How did we get here?
In 2008, Galaxy Diagnostics began commercializing the patented BAPGM (Bartonella alphaproteobacteria Growth Medium) technology. This technology was developed at North Carolina State University’s College of Veterinary Medicine by our co-founders, Dr. Ed Breitschwerdt and Dr. Ricardo Maggi. When Dr. Amanda Elam, now President of Galaxy Diagnostics, joined the company the same year, she brought with her an entrepreneurial drive that helped take diagnostic testing to market.
One year later, in 2009, the Bartonella ePCR method became available to veterinarians for testing animal patients. In recognition of 10 years since this important milestone, we’re kicking off a series of posts about the history of our company and the pathogens we work with.
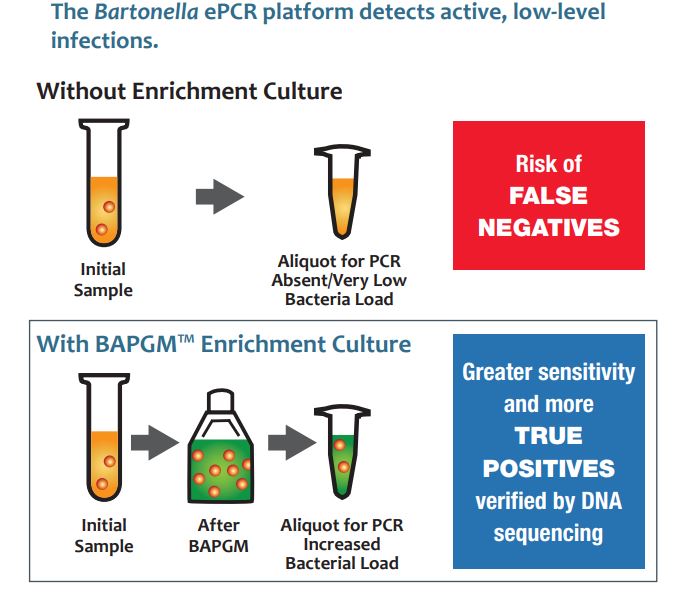
Ten years ago, tests for bartonellosis (Bartonella infection) resulted in higher rates of false negatives and misdiagnosis because of the stealth and anonymity of this emerging pathogen. Bartonella are stealth pathogens that infect accidental hosts at very low levels and grow slowly while causing a spectrum of acute symptoms from the familiar Cat Scratch Disease to lesser-known chronic health problems. Galaxy’s mission from the beginning has been to offer better tests for Bartonella infections and to raise awareness in the medical community to improve patient outcomes.
The bartonellosis testing Galaxy Diagnostics was able to offer with the Bartonella ePCR test brought sensitivity and specificity that had not been available in previous tests. Bartonella ePCR is a two-step process in which the samples are first enriched in BAPGM and then analyzed using PCR and DNA sequencing. Through strategic partnerships with organizations like IDEXX and continued awareness-building, Bartonella ePCR found its place in vector-borne disease diagnostics for animal testing, and Galaxy began to grow.
We now had a test that could help providers diagnose patients who may be suffering from chronic or atypical bartonellosis. In 2012, Galaxy became a CLIA-accredited laboratory and began offering Bartonella testing for humans as laboratory developed tests (LDTs). For the first time, physicians in the United States (except for New York) could order Bartonella ePCR testing for patients.
It usually takes years to translate new knowledge and novel diagnostics for a pathogen into effective clinical practice. Ten years ago, many physicians were unfamiliar with the range of symptoms associated with Bartonella infections. Through our educational efforts and the expertise of our Medical Director Dr. Mozayeni, a key opinion leader in the field, Galaxy Diagnostics was able to shorten this gap. The large growth seen in “Bartonella” diagnostic codes filed shortly after our human health launch is evidence of the success of our focus on medical education efforts and motivates us to continue developing and sharing educational material.
Galaxy has remained “the best in Bartonella testing”, but we have also focused on expanding our test offerings to other vector-borne pathogens in recent years. In 2015, we added PCR testing for common tick-borne pathogens such as Anaplasma, Ehrlichia, Babesia, and Rickettsia to offer physicians more diagnostic data from a single lab rather than having to use several. In 2017, we expanded our menu further to include standard-of-care two-tier testing for Lyme disease. Lyme borreliosis, bartonellosis, and babesiosis often present with similar clinical symptoms, so having reliable tests for each of them is crucial to a proper diagnosis.
“The kindest form of therapy is a more accurate diagnosis.” – Dr. Ed Breitschwerdt
Our goal in 2019 is to continue developing advanced diagnostic methods for tick-borne pathogens. The research and development team has a busy year ahead with the award of a Federal grant to advance Bartonella endocarditis diagnostics as well as ongoing research projects to improve laboratory testing for Borrelia. Furthermore, our researchers continue to publish papers that reveal more about Bartonella and other pathogens. This year is going to be an exciting one, and we look forward to sharing it with you all.