We are very excited to announce the launch of Bartonella Digital ePCR™. This advanced DNA test methodology provides the most sensitive direct detection for Bartonella species infections. Bartonella infections can cause a broad range of diseases, from acute cases of cat scratch disease and trench fever to culture negative endocarditis, rheumatologic disease, neurologic disease, vasoproliferative tumors, and other Bartonella-associated diseases states.
Bartonella Digital ePCR™ provides important advantages over antibody testing and conventional PCR:
- Identifies positive cases missed by IFA serology testing
- Confirms active infection via direct detection of Bartonella DNA
- Offers up to 10x more sensitive than conventional PCR
- Detects a broad range of Bartonella species in one test
Conventional testing for diagnosis of Bartonella infections includes IFA serology and DNA testing by PCR. However, like other flea and tick-borne pathogens, Bartonella often elude host immune response and infect at very low levels in blood. Bartonella Digital ePCR™ combines the power of two technologies to greatly increase the likelihood of DNA detection in patient specimens. Our patented BAPGM enrichment step grows the low abundance organisms present in a patient sample up to detectable levels, while droplet digital PCR (ddPCR) confirms the presence of the target organism via 20,000 PCR reactions instead of a single PCR reaction. The test panel includes ddPCR on both blood and blood culture after BAPGM enrichment.
Published data suggests that Bartonella Digital ePCR™ is up to 10 times more sensitive than our original Bartonella ePCR™ test panel (BAPGM enrichment plus PCR). In this study supported by funding from the National Heart Lung and Blood Institute at the National Institutes of Health, Galaxy scientists reported that ddPCR sensitivity at 47% was significantly better than qPCR at 5.4% on 112 whole blood and BAPGM blood culture samples from patients with chronic symptoms and high levels of animal and vector contact. Consistent with prior research, BAPGM enrichment confirmed by PCR identifies positive cases missed by antibody testing. Further research is needed to confirm clinical utility for specific presentations and disease stages of Bartonella species infection.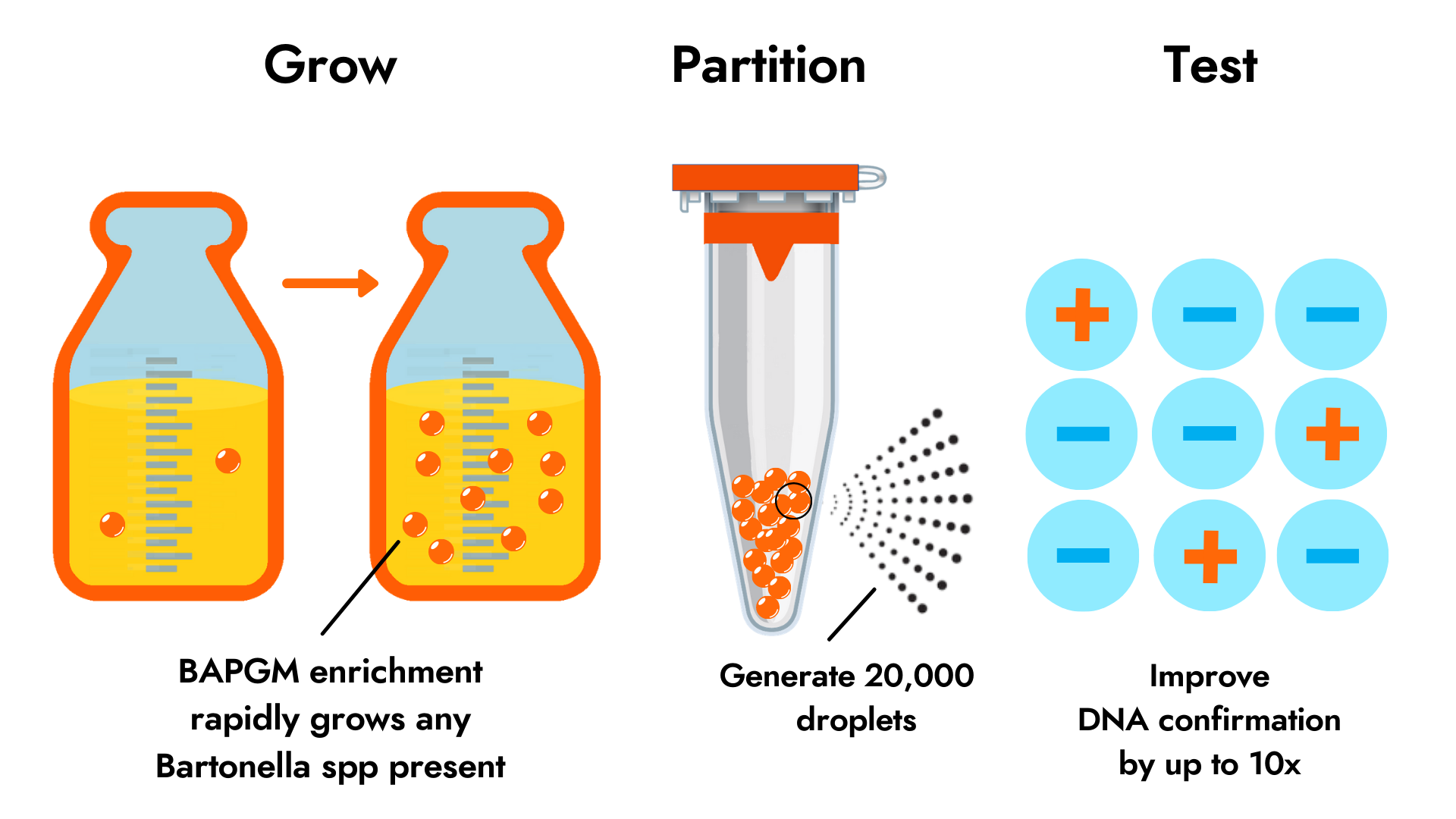 “Advancing the sensitivity of existing test offerings is central to our mission to bring the most scientifically advanced sample enrichment technologies and diagnostic advances to the forefront of flea- and tick-borne disease”, said Galaxy CEO Amanda Elam. “A growing body of published research suggests that Bartonella species infections play a key role in chronic illness, from cancer involvement to heart and liver infections, especially for those individuals with high exposure to fleas, ticks, and animals. We are committed to improving the standard of diagnostic care for detection of these elusive, low abundance pathogens to ensure better patient care for those at highest risk of infection and severe disease globally.”
“Advancing the sensitivity of existing test offerings is central to our mission to bring the most scientifically advanced sample enrichment technologies and diagnostic advances to the forefront of flea- and tick-borne disease”, said Galaxy CEO Amanda Elam. “A growing body of published research suggests that Bartonella species infections play a key role in chronic illness, from cancer involvement to heart and liver infections, especially for those individuals with high exposure to fleas, ticks, and animals. We are committed to improving the standard of diagnostic care for detection of these elusive, low abundance pathogens to ensure better patient care for those at highest risk of infection and severe disease globally.”
As increasingly recommended by medical experts, we recommend a combination baseline testing protocol using Bartonella Digital ePCR™ to confirm active infection and IFA Serology for Bartonella species to detect the presence of antibodies.


