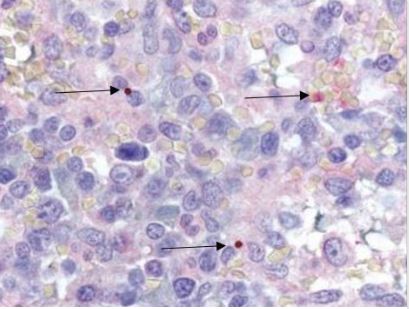
Anaplasma species are bacteria that can evade the antimicrobial defenses of white blood cells, like neutrophil granulocytes, and negatively affect the immune system. One of the methods they use is living inside the cells of the host.
These bacteria cause a condition called anaplasmosis and are similar to other tick-borne stealth pathogens, such as Ehrlichia species. In the past, Anaplasmosis was called “human granulocytic ehrlichiosis” due to morphological and pathogenic similarities between the two species. In 2001 the bacteria was reclassified under a different genus and the medical condition it causes is now known as “human granulocytic anaplasmosis”.
The primary cause of anaplasmosis in the United States and Europe is Anaplasma phagocytophilum. According to the CDC, reported cases in the United States increased by 39% from 4,151 in 2016 to 5,762 in 2017. Most cases are reported in the Northeastern, Midwestern, and Western regions of the United States. The largest amount of new cases were reported in states such as Connecticut, New Jersey, and Maine. However, cases are increasingly reported outside of these areas with the geographic range expanding down into parts of Virginia.
Anaplasma capra was also identified as a potential pathogenic species by researchers in 2018. This species typically infects goats but was identified as the infectious agent causing 6% of patients with history of tick bites to experience non-specific symptoms in Northern China.
Transmission
Anaplasma species are primarily transmitted via bites from infected Ixodes ticks. Ticks become infected when they feed on animals that host the bacteria without experiencing clinical symptoms (reservoirs). The primary reservoir for Anaplasma phagocytophilum in North America is the white-footed mouse, but research suggests that their prevalence is also dependent upon white-tailed deer populations in the Eastern region of the United States. Furthermore, studies have shown that squirrels, voles, and additional deer species can carry these bacteria as well.
The primary tick vector for Anaplasma species is Ixodes scapularis in the Midwestern and Eastern United States. On the west coast, Ixodes pacificus plays a larger role in transmitting these bacteria. According to the European Disease Center for Control and Prevention, the primary vector is Ixodes ricinus across Europe. These tick species can infect humans with co-infections, like Lyme borreliosis or babesiosis, during a single bite. People that spend time in the woods or have outdoor occupations in endemic areas may be at a higher risk of anaplasmosis and other tick-borne illnesses.
Anaplasma species infections can also be attributed to blood transfusions in some cases. In New York, a 78-year old patient passed away two weeks after complications from receiving a blood transfusion containing Anaplasma phagocytophilum. The New York State Department of Health found the bacterial DNA in the donor and patient samples using PCR.
Symptoms
The non-specific and chronic symptoms associated with anaplasmosis often mimic other chronic infections and autoimmune diseases. The clinical presentations can range from mild to severe illness. Being aware of a recent tick attachment and whether Anaplasma is endemic to the region is helpful for diagnosis.
A list of common, but not all, symptoms can be seen below:
- Fever
- Muscle aches
- Malaise
- Headaches
- Neurological symptoms (facial palsy, neuropathy)
- Vomiting
- Coughing
- Diarrhea
The clinical signs for patients who are immunocompromised or have co-infections may be more severe.
References
Centers for Disease Control and Prevention. (2019). Anaplasmosis. Retrieved from: https://www.cdc.gov/anaplasmosis/
Guo, W-P. et al. (2018). Human-pathogenic Anaplasma spp., and Rickettsia spp. in animals in Xi’an, China. PLoS Neglected Tropical Diseases, 12(11), e0006916. doi:10.1371/journal.pntd.0006916 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6258427/
Semenza, J. C., & Menne, B. (2009). Climate change and infectious diseases in Europe. The Lancet Infectious Diseases, 9(6), 365-375. doi:https://doi.org/10.1016/S1473-3099(09)70104-5 https://www.thelancet.com/pdfs/journals/laninf/PIIS1473-3099(09)70104-5.pdf

