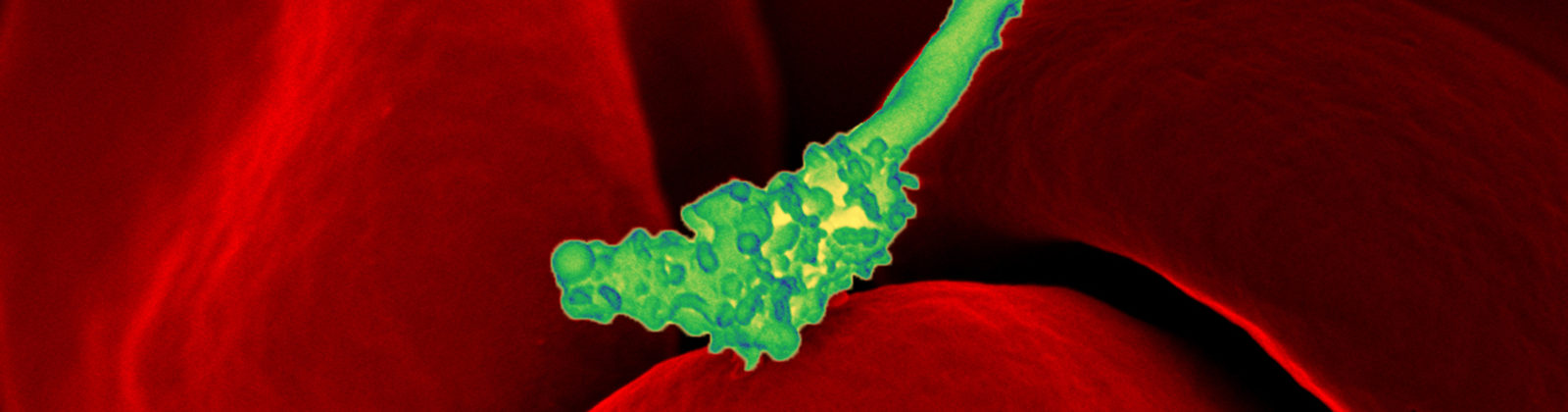
Bartonella species are notoriously difficult to detect when testing patient blood specimens.
The bacteria use immunosuppression as a tactic to avoid immune recognition, so serology tests that detect antibodies can be negative even during a long-standing infection. Furthermore, the bacteria move in and out of the blood in a relapsing pattern. Therefore, a PCR test designed to detect Bartonella species DNA in blood could be positive for a sample collected on one day and then negative on the next day even in blood collected from the same patient.
We’ve previously written about serology and PCR.
Bartonella bacteria adhere to and invade erythrocytes, that is, red blood cells. Diagnostically, how is it possible that a key niche for the bacteria is in the blood, and yet the bacteria often cannot be found there?
“Bartonella species and erythrocyte invasion remains incompletely understood,” said Dr. Ed Breitschwerdt, co-founder of Galaxy Diagnostics. “B. bacilliformis is an outlier in the context of a species that induces a severe, often acute hemolytic anemia. Also, laboratory studies with rodents don’t necessarily recapitulate what happens in larger animals, including humans.” Nonetheless, Dr. Breitschwerdt and other researchers conclude that infection with Bartonella species should be considered in anemia of unknown origin.
Severe Cases and Anemia
As mentioned by Dr. Breitschwerdt, there is one species of Bartonella that is an outlier in this puzzle. Bartonella bacilliformis, a species endemic to South America and primarily transmitted by sand flies, is particularly adapted to entering erythrocytes for reasons that are unknown. This species causes Carrion’s disease, characterized by skin nodules and fever, with the acute stage of the infection being called Oroya fever. During the acute phase, there is a rapid rise in fever, sometimes accompanied by anemic shock and death. No one is certain why B. bacilliformis is so much more adept at infecting erythrocytes, but it may have something to do with its double flagella. This whip-like tail controls movement of the bacterium in liquid medium and also plays a role in interacting with the immune system.
In the Lab: Small Animals and Cell Cultures
Keeping in mind Dr. Breitschwerdt’s caution about how laboratory studies do not always apply to people, nonetheless we don’t have a lot of other places to turn for information. Research shows that this is what happens when rodents are experimentally infected with Bartonella species:
- First, the infection is generally cleared from the bloodstream rapidly as the bacteria finds other niches.
- At this point of initial infection, Bartonella species are generally not able to infect erythrocytes.
- Bacteria multiply in other niches. Only after increasing in numbers and developing immune protection are they able to return to the blood stream and adhere to and enter erythrocytes.
- They are more adept at infecting new erythrocytes than mature erythrocytes
In human cell cultures, Bartonella henselae have been recorded entering human erythrocytes within 72 hours of infection. At this point, their ability to avoid immune detection far exceeds comparator flagellates such as Salmonella species. Nonetheless, their replication is somewhat hampered. In laboratory studies, researchers have found that the bacterial count stabilizes at eight bacteria per erythrocyte.
The fact that most Bartonella species have trouble entering mature erythrocytes may be life-saving. It appears that most infection occurs in newly formed cells called reticulocytes, a developmental stage that only lasts about a day before they develop into mature erythrocytes. Overcoming this limitation may be part of what allows B. bacilliformis to infect mature erythrocytes, resulting in Oroya fever, hemolytic anemia and a more dramatic hematological disease than is associated with most other Bartonella infections.
Current Thinking on Erythrocyte Infections in Humans
The host response probably has a large influence on whether and to what degree an individual’s Bartonella species infection develops in erythrocytes. Immune factors influence whether the bacteria are detected in the blood when it is vulnerable as it adheres to an erythrocyte. Some level of bacterial destruction will happen during attempted erythrocyte invasion.
Another factor that is not well understood is infection of the progenitor cells that make erythrocytes. It may be that Bartonella species are not good at invading erythrocytes at all, but rather originate in these progenitor cells. One study showed that CD34+ progenitor cells isolated from humans could be colonized by Bartonella henselae and result in a persistent intraerythrocytic infection. Currently, B. henselae is the only species known to infect CD34+ progenitor cells.
Lastly, a well-functioning lymph system, including the spleen, continually clears infected erythrocytes.
Read more about bartonellosis, lymph nodes and the spleen.
It may be that bacteria found in the blood don’t necessarily represent an unusual blood event but rather the disease process affecting the immune system. When the immune system is functioning normally, detectable Bartonella bacteria in the bloodstream may or may not be related to infection of erythrocytes. Rather, bloodstream infection may represent the periodic release of bacteria from other areas of the body.
Conclusion
Despite laboratory bench research and small animal experiments, bloodstream availability of Bartonella species bacteria in humans is incompletely understood. What is known is that despite the ability of bacteria to enter erythrocytes, they are rarely found in circulating erythrocytes in the bloodstream.
References
Deng, H. et al. (2018). Molecular mechanisms of Bartonella and mammalian erythrocyte interactions: A review. Frontiers in Cellular and Infection Microbiology, 8, 431. doi:10.3389/fcimb.2018.00431 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6299047/
Pitassi, L. H. et al. (2007). Bartonella henselae infects human erythrocytes. Utrastructural Pathology, 31(6), 369-372. doi:10.1080/01913120701696510 https://www.ncbi.nlm.nih.gov/pubmed/18098053
Vieira-Damiani, G. et al. (2016). Bartonella henselae initial infection of mature human erythrocytes observed in real time using bacterial endogenous fluorescence. Journal of Tropical Diseases and Public Health, 4(2), 207. doi:10.4172/2329-891X.1000207 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5639914/
McCullough, J. (2014). RBCs as targets of infection. Hematology, 2014(1), 404-409. doi:10.1182/asheducation-2014.1.404 https://www.ncbi.nlm.nih.gov/pubmed/25696886
Hong, J. et al. (2017). Lymphatic circulation disseminates Bartonella infection into bloodstream. Journal of Infectious Diseases, 215(2), 303-311. doi:10.1093/infdis/jiw526 https://www.ncbi.nlm.nih.gov/pubmed/27803173
Mändle, T. et al. (2005). Infection of human CD34+ progenitor cells with Bartonella henselae results in intraerythrocytic presence of B henselae. Blood, 106(4), 1215-1222. doi:10.1182/blood-2004-12-4670 https://ashpublications.org/blood/article/106/4/1215/21539/Infection-of-human-CD34-progenitor-cells-with