The most sensitive test method available for confirmation of Bartonella spp infections.
Bartonella are elusive bacteria, evading immune response and direct detection by hiding in cells and tissue. Based on NCSU research studies, many patients with detectable Bartonella spp DNA never develop detectable antibodies, while others may only show very low titers. The result is many patients with active Bartonella infection are missed by both conventional DNA and antibody testing.
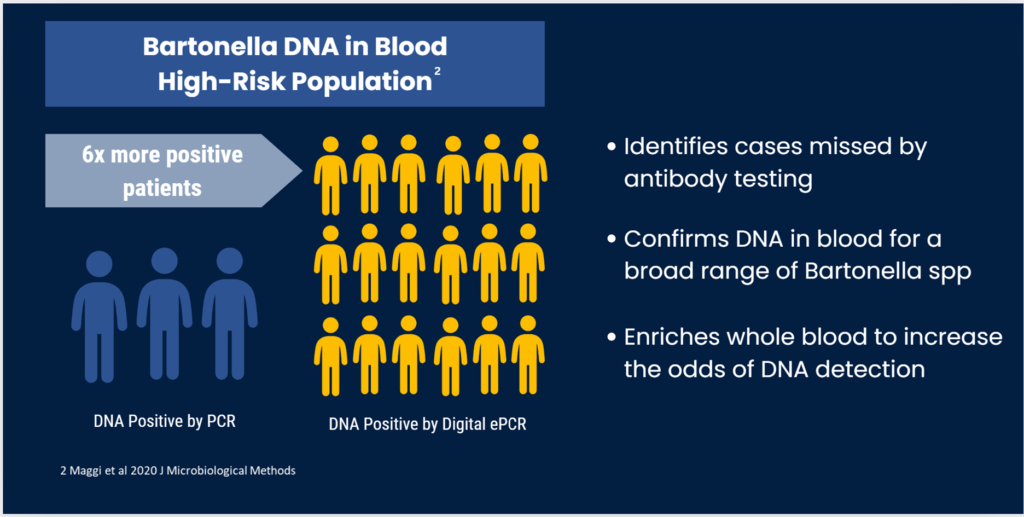
Our solution is Bartonella Digital ePCR™, a DNA detection approach that increases the number of patients confirmed by up to 6 times compared to conventional detection methods, like qPCR, NGS, and other direct detection methods.
Over 20 species of Bartonella have now been documented in human and animal diseases. We use genus level primers in our that are designed to pick up a broad range of Bartonella species. This approach ensures that positive cases of infection with less common Bartonella species are not missed.
How the Test Works
Our Bartonella Digital ePCR™ test is powered by two revolutionary technologies, sample enrichment using our proprietary Bartonella alpha Proteobacteria Growth Medium (BAPGM) followed by DNA confirmation by droplet digital PCR.
Bartonella Digital ePCR™ requires 5 ml of whole blood which is put into BAPGM liquid culture for one week. We confirm the presence of Bartonella spp DNA in both the whole blood and in the BAPGM whole blood culture by droplet digital PCR.
This approach can confirm up to 6x more Bartonella-infected patients than standard qPCR (n=38 high risk patients), supporting earlier diagnosis of infection.
Increased Sensitivity of Proprietary BAPGM Sample Enrichment
Bartonella species are often able to evade detection by even the most sensitive test methods due to the low abundance of organisms present in blood samples. We overcome this diagnostic challenge by growing any bacteria present in our proprietary BAPGM enrichment culture. This sample enrichment step increases the bacterial load in patient samples up to detectable levels for PCR testing.
Increased Sensitivity of Digital PCR
Looking for microbial DNA in a blood sample is like looking for a need in a haystack. Conventional direct detection techniques often miss confirmation of low abundance pathogens due to inhibitory factors, like host DNA, that are also present. Digital PCR technologies overcome this limitation by partitioning the sample into ~20,000 droplets and performing a PCR reaction on each droplet. Based on our SBIR-funded research, this powerful technology increases the sensitivity of PCR detection by as much as 10 times.
Test Recommendations
Along with a growing community of medical researchers worldwide, we recommend maximizing diagnostic data by testing for both antibodies and DNA evidence of infection. Bartonella are elusive bacteria, evading immune response by hiding in cells and tissue. Based on NCSU research studies, many patients with detectable Bartonella spp DNA never develop detectable antibodies, while others may only show very low titers.
Learn more about when and why to order Bartonella IFA Serology testing from our Chief Scientific Officer, Ed Breitschwerdt, DVM, DACVIM, globally recognized as a leading expert on bartonellosis and other vector borne infections.
FAQS
What is the recommended use of this test?
For newly infected patients, we recommend testing for both DNA and antibody evidence of Bartonella species infection. For patients who have failed treatment or who have recently undergone aggressive antibiotic therapy, direct detection using this platform may not be as helpful.
Is Triple Draw testing still recommended?
Prior research suggests that serial testing on three samples collected in a one week period can increase the odds of confirming a positive case of Bartonella species infection. Future studies are required to quantify potential gains of serial testing for this more sensitive Bartonella Digital ePCR™ test panel.
Which species are detected?
The Bartonella Digital ePCR™ platform will only confirm whether Bartonella species were detected or not. It will not differentiate between species. It was validated using four of the most common species of Bartonella infection in the United States (B. henselae, B. quintana, B. vinsonii berkhoffii, and B. koehlerae).
Does this test method confirm active infection?
Yes, a positive result indicates an active infection. However, the determination of whether the presence of the organism is causing the patient’s symptoms is up to the clinician.
What is the price?
Bartonella Digital ePCR™ relies on innovative technologies that are currently not covered by insurance companies. As is common for new test methods, it is currently available on a self-pay basis only. Please see our full test menu and pricing here.



