The most sensitive and specific form of Bartonella testing available for confirmation of active infection.
Works for a broad range of Bartonella species
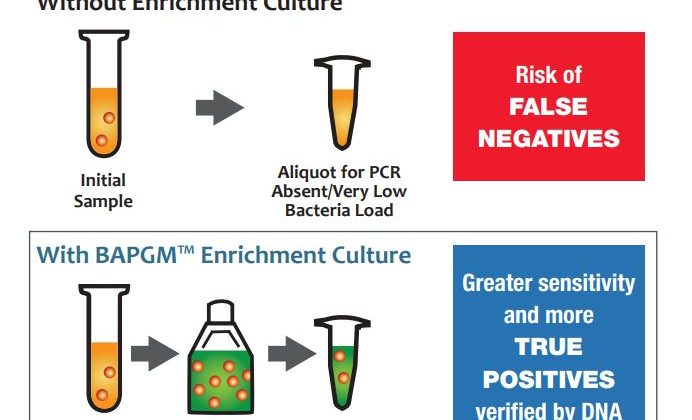
- Patented BAPGM Enrichment Step.
Even the most sensitive DNA detection methods can produce false negatives due to extremely low levels of DNA in a given sample. We overcome this testing limitation by enriching samples for one week in a patented enrichment media called BAPGM (Bartonella Alpha Proteobacteria Growth Medium), which increases the bacterial load in patient samples up to detectable levels for PCR testing. - Pre- and Post-enrichment PCR.
The Bartonella ePCRTM platform is designed to minimize the likelihood of false negatives across all types of samples and patient cases. We run PCRs both before and after enrichment culture to ensure that we capture any viable or nonviable DNA present in the sample. - DNA Sequence Verification.
We sequence verify all positive PCR results to identify the species of infection, ensuring the highest level of specificity achievable. Sequence identification is an important step for Bartonella detection, as virulence and treatment resistance may vary across species.
Our Bartonella ePCRTM platform was developed by Dr. Ed Breitschwerdt and Dr. Ricardo Maggi at North Carolina State University’s College of Veterinary Medicine. The sensitivity and specificity of our test method and key indications for testing are supported extensively by peer-review publications. Importantly, we continue to investigate the medical importance of Bartonella infection for human and animal medicine with research collaborators at leading research institutions.
Bartonella ePCRTM Overcomes Limitations of Conventional Testing for Bartonellosis
Current CDC guidelines recommend three possible approaches for the confirmation of a Bartonella infection:
- DNA testing – Bartonella infects at exceedingly low levels that are below the limit of detection for DNA test methods. DNA tests can only detect the bacteria if at least one or two copies make it into the small sample tested. The result is a test with high false negative rates.
- Antibody testing – Bartonella often evade immune response, resulting in high false negative results for IFA serology due to a lack of antibodies. IFA testing also involves a risk of false positive due to potential cross reactivity with other microbes. Importantly, IFA antigens are currently limited to 2 or 3 of the most common species of infection, while 17 species are currently associated with disease in humans and animals.
- Plate culture – Bartonella is highly fastidious and notoriously difficult to grow using traditional bacterial culture methods, which can take 4 to 6 weeks with a very low probability of a positive result. This method is primarily reserved for bacterial isolation and susceptibility testing in research labs.
All three test methods involve significant testing limitations.
For more information: