Skin is the largest human organ. It is also the first place that flea and tick-borne pathogens encounter the human immune system. While some skin symptoms are signature markers for specific pathogens or families of pathogens, others provide hard-to-interpret clues that can be linked to a wide variety of diseases. Lyme borreliosis and bartonellosis are both associated with specific as well as non-specific symptoms involving the skin.
Vector-borne pathogens have evolved to evade the host’s immune response in the skin. This evolution can make them more infectious when they enter the body, but it can also interfere with evolution that optimizes for systemic infection.
For example, in mice, a gene that allows Borrelia burgdorferi to replicate quickly during initial skin infection has been shown to lead to higher bloodstream load and more bacteria far from the initial tick bite. This suggests that the bacteria, a cause of Lyme disease, has been made more pathogenic by this genetic variation.
On the other hand, research suggests that some Bartonella species strains developed to be better suited to evade the host’s immune system in erythrocytes (a type of blood cell). In parallel evolution, other strains became better suited to evade the host’s immune system in skin cells.
Regardless of how they have evolved, these first encounters with the skin can provide signature symptoms for the diagnosis of some infections.
Lyme Borreliosis
The bullseye rash (erythema migrans) that some people experience upon becoming infected with Borrelia burgdorferi is an interaction between the immune system, the tick bite, and the bacteria.
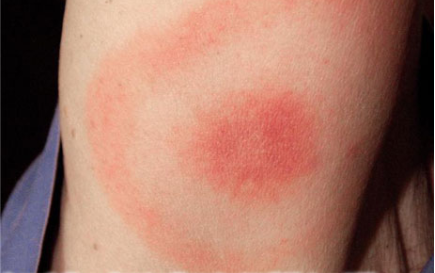
The center of the rash is an immune reaction to tick saliva. The outer circle is an immune reaction to the bacteria. The erythema migrans rash may look different in different people – or may not be seen at all – because of differences in the immune system response between various hosts.
Late-stage untreated Borrelia burgdorferi infection can also cause a skin condition called acrodermatitis chronica atrophicans. It generally appears in the limbs, causing swelling and then discoloration and peeling of the skin that is sometimes characterized as looking like cigarette paper. Treatment with antibiotics can stop the disease process, but the skin discoloration may be permanent.
Bartonellosis
Bartonella henselae and Bartonella quintana are associated with a skin condition called bacillary angiomatosis, which is most commonly seen in people who have a Bartonella infection and also a compromised immune system from a cause such as HIV infection. Bacillary angiomatosis is recognizable by nodules that form on or under the skin. The same disease process can also create nodules in other organs of the body. If they break the skin or press against bone or other sensitive areas, they can cause pain. Bacillary angiomatosis was documented for the first time in a patient infected with Bartonella elizabethae.
More controversially, skin striae are associated with Bartonella species infection. These marks look similar to stretch marks and have been associated anecdotally with Bartonella species infection. Dr. Marna Ericson at the University of Minnesota Medical School is working on further research to test skin samples and establish the preliminary research needed to do larger studies of the association between skin striae and Bartonella infection. You can find out more about her work here.
Galaxy Diagnostics is involved with Dr. Ericson’s work. In 2016, Dr. Ericson published a description of biopsies of Bartonella species-associated striae from two patients. These biopsies found the striae to have a higher bacterial load than surrounding skin, and the marks had disrupted vasculature.
Non-Specific
Vector-borne diseases may have further effects on the skin that are not fully understood. A study of all Lyme disease patients diagnosed in the Netherlands between 1986 and 2016 found that Lyme disease was not associated with increased mortality, but patients did have more cases of non-melanoma skin cancers.
Finally, vector-borne infections can lead to skin changes that are signs of an active or overactive immune system. These changes don’t necessarily point to a particular pathogenic infection or to any infection at all. For example, granulomas are lesions found in the skin and other organs that can be associated with an infection or with the autoimmune disease sarcoidosis.
Researchers have not found an association between granulomas and flea- and tick-borne diseases. Nonetheless, individual cases in which an association is suspected do pop up in peer-reviewed journals. For example, one person with a family history of sarcoidosis developed granulomas after becoming infected with Bartonella henselae.
Considering how important skin is to the disease process of many vector-borne infections, it is no wonder that research is underway to improve the skin’s defenses. For example, researchers are looking at an antibiotic product to apply to the skin to prevent Lyme disease.
Conclusion
Skin is the first layer of defense against an environment full of bacteria, viruses, and other pathogens that can negatively impact human health. As is the case with irritants such as pollen or mold, one individual’s skin may be more susceptible to vector-borne pathogens than another’s. Furthermore, a species that a patient is exposed to may be adapted to infiltrating the skin. It is important to regularly check your skin for changes and visit a physician if you think you have been exposed to flea- and/or tick-borne pathogens.
References
Aranjuez, G. F. et al. (2019). Borrelia burgdorferi bbk13 is critical for spirochete population expansion in the skin during early infection [Published online]. Infection and Immunity, 87(5). doi:10.1128/IAI.00887-18 https://iai.asm.org/content/early/2019/02/14/IAI.00887-18.long
Deng, H. et al. (2018). Molecular mechanisms of Bartonella and mammalian erythrocyte interactions: A review [Published online]. Frontiers in Cellular and Infection Microbiology, 8. doi:10.3389/fcimb.2018.00431 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6299047/
Griffin, A. H. (2018). The bulls-eye rash of Lyme disease: Investigating the cutaneous host-pathogen dynamics of erythema migrans. Available at the American Society for Microbiology website: https://www.asm.org/Articles/2018/April/going-skin-deep-investigating-the-cutaneous-host-p
Marques, A. et al. (2018). Transcriptome assessment of erythema migrans skin lesions in patients with early Lyme disease reveals predominant interferon signaling. The Journal of Infectious Diseases, 217(1), 158-167. https://doi.org/10.1093/infdis/jix563 https://academic.oup.com/jid/article/217/1/158/4584510
Moniuszko-Malinowska, A. et al. (2018). Acrodermatitis chronica atrophicans: Various faces of the late form of Lyme borreliosis. Postepy Dermatologii I Alergologii [Advances in Dermatology and Allergology], 35(5), 490-494. doi:10.5114/ada.2018.77240 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6232541/
Koehler, J. E. (1997). HIV and Bartonella: Bacillary angiomatosis and peliosis. Available at the HIVInSite website: http://hivinsite.ucsf.edu/InSite?page=kb-05-01-03
Julieta, C. et al. (2019). First report of bacillary angiomatosis by Bartonella elizabethae in an HIV-positive patient [Epub ahead of print]. American Journal of Dermatopathology. Doi:10.1097/DAD.0000000000001439 https://www.ncbi.nlm.nih.gov/pubmed/31094718
Maluki, A. et al. (2016). 514 non-traditional skin striae as a manifestation of bartonellosis. Journal of Investigative Dermatology, 136(5) (Suppl. 1), S90. https://doi.org/10.1016/j.jid.2016.02.551 https://www.jidonline.org/article/S0022-202X(16)30605-4/fulltext
Obel, N. et al. (2018). Long term survival, health, social functioning, and education in patients with European Lyme neuroborreliosis: Nationwide population based cohort study. BMJ (Clinical Research), 361, k1998. doi:10.1136/bmj.k1998 https://www.ncbi.nlm.nih.gov/pubmed/29848547
Morgado, F. et al. (2018). Erythema nodosum and sarcoid granulomas – Letting the cat out of the bag. Dermatology Online Journal, 24(12), 3. https://escholarship.org/uc/item/2f31g4pv
Jin, N. et al. (2019). Maximum loaded amorphous azithromycin produced using the wetness impregnation method with fractional steps for dermal prophylaxis against Lyme disease. Die Pharmazie, 74(6), 345-349. doi:10.1691/ph.2019.8175 https://www.ncbi.nlm.nih.gov/pubmed/31138371


