In the United States as testing problems mounted for the novel coronavirus (SARS-CoV-2, the virus that causes COVID-19), tick-borne disease patients recognized something they have experienced.
Physicians rely on clinical symptoms as well as diagnostic testing to make treatment decisions. The more accurate a test is for a specific disease then the more certain a physician can be when making a diagnosis and beginning treatment. This ultimately results in a better outcome for the patient.
When testing is limited or inaccurate, public health and clinical decisions become much more difficult. A recent study found that the FDA-approved and CDC recommended two-tier testing protocol for Lyme disease missed 70% of cases where patients had an erythema migrans (EM) rash greater than 5 cm in diameter. The consequence has been dramatic both for individual patients and for obtaining public health resources for tick-borne disease.
But what happens when there is no test available at all? This was the challenge when SARS-CoV-2 appeared. The pandemic is exposing many of the challenges facing development and commercialization of new tests. Point of care (POC) diagnostics are ideal during public health emergencies because the assay is usually very simple to perform, and test results are available almost immediately. Examples of POC tests that are often used include a rapid strep test, a quick UTI screening test, or a rapid flu test in your doctor’s office.
However, for newly discovered pathogens like SARS-CoV-2, these assays are not immediately available meaning new ones must be developed, validated according to regulatory standards, and then offered commercially by approved laboratories with the appropriate equipment. Developing the test and acquiring the necessary reagents and supplies is a difficulty many laboratories face. In the race to develop a SARS-CoV-2 test, these difficulties had enormous consequences for the United States.
The issue began with the Center for Disease Control (CDC) providing local and state public health laboratories with diagnostic kits that could be used to run their FDA-approved assay on potential SARS-CoV-2 samples sent in by healthcare providers. Far fewer kits were distributed than state public health labs needed, and it was soon discovered that results were unreliable due to faulty reagents.
A test protocol used by the CDC was then made available for commercial and government laboratories to create their own laboratory developed tests (LDTs). However, validation of assays under the FDA and/or CLIA regulatory processes can take weeks and requires hard-to-find positive control samples, which extends the amount of time before healthcare providers can access reliable tests for potentially infected patients. Furthermore, news reports suggested that laboratories were having a hard time acquiring the necessary reagents and supplies for test development because manufacturers were overburdened by the global demand.
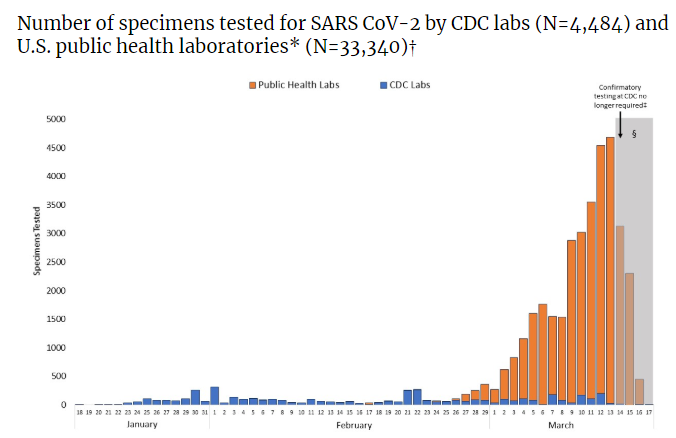
To shorten that development, the FDA has released a list of approved equipment substitutions and validation that can be piggybacked on existing validation work during the public health emergency. Commercial laboratories have now begun offering testing in the United States, alleviating the pressure on the public laboratories to keep up with demand. Up to date information about the current testing situation in the United States can be found here.
Conclusion
Accurate and accessible testing is increasingly necessary for emerging zoonotic diseases as shown by the ongoing coronavirus pandemic. Novel varieties of pathogens as well as supply chains and development issues can slow availability of tests. At the same time, these tests are needed now more than ever to determine the best route for patient care. They are also crucial for disease surveillance, assessing the true mortality and morbidity, determining appropriate resource allocation, and much more.
At Galaxy Diagnostics, our focus remains on testing for flea- and tick-borne diseases linked to acute and chronic illness. While we understand the urgent need for SARS-CoV-2 testing, we are leaving this expertise up to the virologists at state public health labs and at the big labs that can offer high volumes of testing on high-throughput systems for the most rapid turnaround of test results. In the meantime, we will continue to provide services with social distancing measures to protect our employees and local community.
References
Jacobs, A. (2020, February 24). New genomic tests aim to diagnose deadly infections faster. New York Times. Available at:
https://www.nytimes.com/2020/02/24/health/genomic-diagnostic-tests.html
Food and Drug Administration. (2020). Policy for diagnostic tests for coronavirus diseas-2019 during the public health emergency [Docket number FDA-2020-D-0987]. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/policy-diagnostic-tests-coronavirus-disease-2019-during-public-health-emergency?utm_campaign=2020-03-16%20COVID-19%20Diagnostics%20FDA%20Updates%20Policy&utm_medium=email&utm_source=Eloqua
Centers for Disease Control and Prevention. (2020). Coronavirus disease 2019 (COVID-19) COVID-19 situation summary. Available at: https://www.cdc.gov/coronavirus/2019-nCoV/summary.html
Centers for Disease Control and Prevention. (2020). Coronavirus disease 2019 (COVID-19) testing. Available at: https://www.cdc.gov/coronavirus/2019-ncov/about/testing.html
Food and Drug Administration. (2018). Laboratory developed tests. Available at: https://www.fda.gov/medical-devices/vitro-diagnostics/laboratory-developed-tests
Food and Drug Administration. (2017). Discussion paper on laboratory developed tests. Available at: https://www.fda.gov/media/102367/download
Jordans, F. (2020, March 9). Experts: Rapid testing helps explain few German virus deaths. Yahoo! News. Available at: https://news.yahoo.com/experts-rapid-testing-helps-explain-172747085.html?guccounter=1
Johnson, C. Y. et al. (2020, February 25). A faulty CDC coronavirus test delays monitoring of disease’s spread. The Washington Post. Available at: https://www.washingtonpost.com/health/2020/02/25/cdc-coronavirus-test/
Harris, R. (2020, March 11). No guarantee you’ll get tested for COVID-19, even if your doctor requests it. NPR. Available at: https://www.npr.org/sections/health-shots/2020/03/11/814189027/no-guarantee-youll-get-tested-for-covid-19-even-if-your-doctor-requests-it?utm_source=facebook.com&utm_campaign=npr&utm_medium=social&utm_term=nprnews&fbclid=IwAR1f_aNJOFRrpHhfg3VqMxesht1V-c90tniQyDnHsFWs1I9aS1Wu-8Fq96Q


