Bartonellosis is a term used to encompass all infections caused by pathogenic Bartonella species. Bartonella are emerging, flea-borne bacteria that are highly adapted to living in mammalian hosts and are implicated in a wide spectrum of diseases in humans and animals.
| Bartonella species | Primary Reservoir | Vector | Accidental Host(s) and [human disease] |
|---|---|---|---|
| Bartonella alsatica | Rabbit (Oryctolagus cuniculus) | Rabbit flea? (Spilopsyllus cuniculi) | Human [endocarditis] |
| Bartonella bacilliformis | Human | Sandfly (Lutzomia verrucarum) | None [Carrion's disease, Oroya fever, verruga peruana] |
| Bartonella clarridgeiae | Cat (Felis catus) | Cat flea (Ctenocephalides felis) | Human, dog [cat scratch disease, endocarditis] |
| Bartonella elizabethae | Rat (Rattus norvegicus) | Oriental rat flea (Xenopsylla cheopis) | Human, dog [endocarditis, neuroretinitis] |
| Bartonella grahamii | Wild mice (agrestris, Apodemus, Clethrionomys glareolus, Microtus flavicollis) | Rodent fleas | Human [neuroretinitis] |
| Bartonella henselae | Cat (felis catus) | Cat flea (Ctenocephalides felis) | Human, dog, horse, marine animals [Cat scratch disease, bacillary angiomatosis, endocarditis, neuroretinitis, bacteraemia] |
| Bartonella koehlerae | Cat (Felis catus) | Cat flea (Ctenocephalides felis) | Human, dog [endocarditis] |
| Bartonella melophagi | Sheep (Ovis aries) | Sheep ked (Melophagus ovinus) | Human [pericarditis, chronic fatigue] |
| Bartonella quintana | Human | Body louse (Pediculus humanis) | Cat, dog [endocarditis, trench fever, bacillary angiomatosis] |
| Bartonella rochalimae | Canids | Fleas? (Pulex irritans, Pulex stimulans) | Human, dog [bacteraemia, fever] |
| Bartonella tamiae | Unknown (Rat?) | Mites? Ticks? | Human [bacteraemia, fever] |
| Bartonella vinsonii arupensis | White-footed mouse (Peromyscus leucopus) | Unknown (Fleas? Ticks?) | Human [bacteraemia, fever, endocarditis] |
| Bartonella vinsonii berkhoffii | Coyote (Canis latrans) Dog (Canis familiaris) | Unknown (Ticks?) | Human, cat [endocarditis] |
| Bartonella washoensis | Californian ground squirrel (Spermophilus beecheyii) | Fleas (Oropsylla montana) | Human, dog [myocarditis, endocarditis] |
Adapted from: Edward Bealmear Breitschwerdt; Bartonellosis: One Health Perspectives for an Emerging Infectious Disease, ILAR Journal, Volume 55, Issue 1, 1 January 2014, Pages 46–58, https://doi.org/10.1093/ilar/ilu015
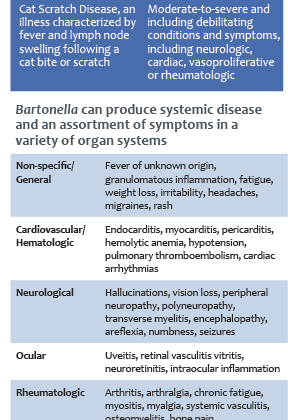
As a genus, these bacteria cycle from the blood to tissues around the body making them difficult to detect and identify. There were only 2 named Bartonella species prior 1990, but now there are about 20 species associated with human disease. Bartonella henselae, the causative agent of Cat Scratch Disease (CSD), is the most common species found in both animals and humans in the United States. Although the first case was described in the 1950s, B. henselae was not identified until extensive research on HIV patients was performed in the 1990s.
Bartonellosis was originally thought to primarily affect immunocompromised individuals because a normal immune system could self-regulate the infection. However, recent research has suggested that immunocompetent people can be chronically infected, but be asymptomatic or show atypical symptoms. Furthermore, these chronic infections can lead to cardiac and neurologic complications that are life threatening. Read on to learn more about symptoms, risk factors, and the testing options that Galaxy Diagnostics offers for Bartonella.
Acute vs. Chronic Bartonellosis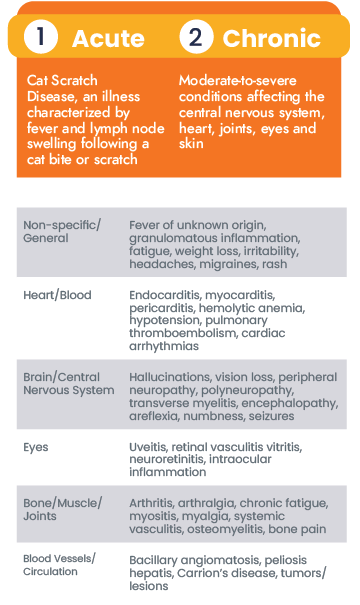
Research on Bartonella species has increasingly suggested its role in chronic diseases ranging from neurovascular to rheumatologic.
Despite these findings, reported cases are often focused on acute infections, such as CSD, Trench Fever, and Carrion’s disease, that show typical clinical signs making them easier to diagnose. Acute infections are likely to be diagnosed and treated quickly, but the story is different for chronically ill patients that are atypical or asymptomatic.
Chronically ill patients may show non-specific symptoms, like headaches, malaise, or fatigue, in the beginning of the infection. As the Bartonella species causes inflammation in different tissues around the body, symptoms can resolve and relapse in a cyclic pattern. Furthermore, co-infections have been been documented with other bacteria (Borrelia burgdorferi, Anaplasma, etc.) and parasites (Babesia microti, Theileria) which complicates clinical diagnosis of chronic bartonellosis even more. Unfortunately, these chronic infections are often mistaken for other disorders, such as immune-mediated diseases (lupus, multiple sclerosis, etc.), and lead to additional economic and health concerns. Patients who were diagnosed after unknowingly struggling with bartonellosis shared their stories to help shed light on the complexity of these diseases.
So what does Bartonellosis look like?
That can be a hard question to answer for researchers, physicians, and specialists alike. Bartonellosis can present in many ways, and atypical manifestations make it even harder to accurately describe clinical patterns. Nevertheless, Bartonella infection is successfully diagnosed in patients all over the world, and awareness of the classic presentation of the disease can help guide diagnosis. There are classic signs that make some cases easier to diagnose than others.
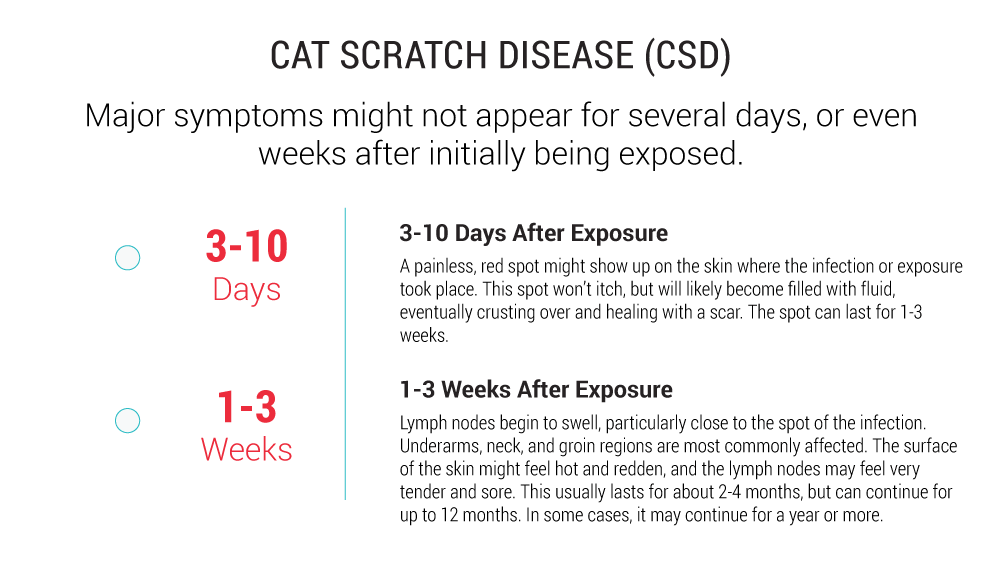
Cat Scratch Disease (Bartonella henselae)
Cat Scratch Disease (CSD) is the most common Bartonella infection diagnosed in humans and animals across the United States. Bartonella henselae, the causative agent, is a flea-borne bacterium that is transmitted between mammal reservoirs through arthropod vectors, like biting flies and ticks. The primary reservoir animal for this species is a cat, but has also been found in horses, dogs and wild animals, such as bats. CSD is typically diagnosed after a scratch from a cat, hence the name, but in some cases patients cannot recall a recent scratch or bite.
Trench Fever (Bartonella quintana)
Trench fever is a common manifestation of bartonellosis that is primarily transmitted by human body lice (Pediculus humanus humanus). It is best known for causing fever, headache, and leg pain in over one million soldiers in Europe during World War I. These soldiers were subjected to poor hygiene and lack of cleanliness that created a breeding ground for arthropod vectors such as body lice. Although trench fever has been around for a long time, the causative agent, Bartonella quintana, was only isolated in 1960 by J.W. Vinson. Currently, B. quintana infection is a higher risk for people who are homeless and more likely to be exposed to infected lice. Mammalian transmission of this pathogen is also possible and was documented in 2007 when a North Carolina woman tested positive after being bitten by a feral cat.

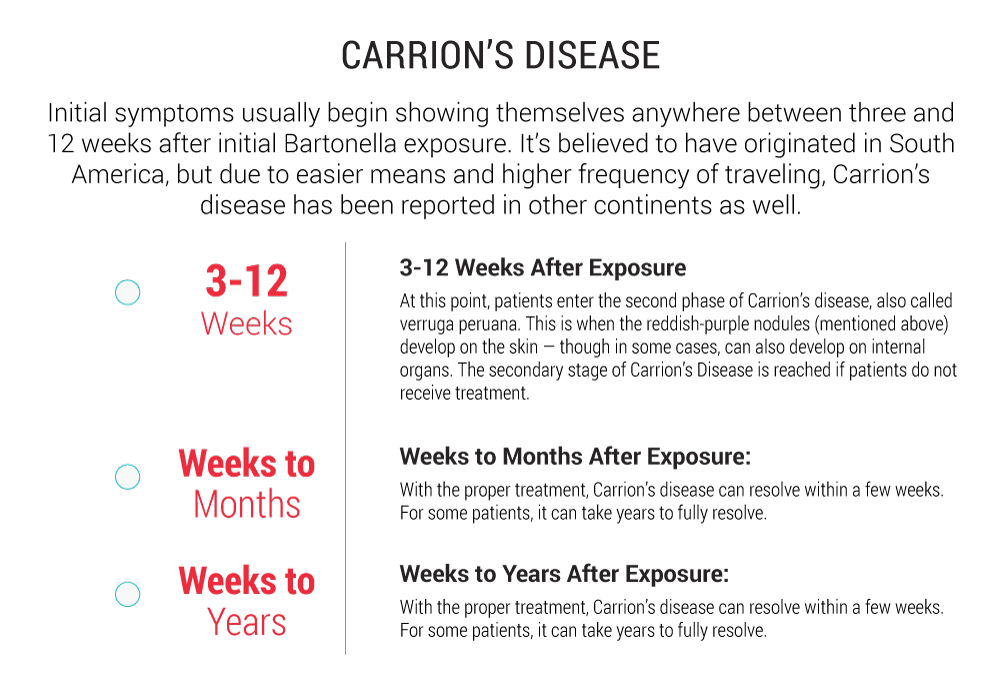
Carrion’s Disease (Bartonella bacilliformis)
Carrion’s disease is named after a Peruvian medical student named Daniel Carrion. Carrion inoculated himself with fluid from verruga peruana lesions in order to document the symptoms that followed, but he died five weeks later. In 1909, a researcher named Alberto Barton was the first to publish Bartonella bacilliformis as the causative agent of Carrion’s disease. It is transmitted by infected sand flies (Lutzomia verrucarum) that bite human hosts primarily in Peru, Ecuador, and Columbia. Despite being geographically isolated, cases have also been described in travelers to South America months to years after returning to non-endemic countries. This infection often presents itself as an acute illness, referred to as Oroya fever, as well as a chronic condition called verruga peruana (Peruvian warts).
Bartonella Endocarditis
Endocarditis is inflammation of the inner lining and valves of the heart, typically caused by the presence of bacteria or a viral pathogen. Endocarditis typically presents with non-specific clinical signs such as chills, fatigue, or muscle pain, but can have devastating long-term effects that may require surgery to replace the valves. Death may result if endocarditis is not diagnosed and treated early enough. When imaging shows the disease, physicians will collect samples to culture so a causative pathogen can be identified.
Culture-negative endocarditis, in which a pathogen cannot be readily isolated, represents up to 31% of all human cases. When this is the case, Bartonella species are often the culprit. Since 1993, a wide spectrum of Bartonella species have increasingly been implicated in endocarditis in humans and dogs. Nine Bartonella species in humans and seven Bartonella species in dogs have been identified as the pathogenic bacteria for endocarditis. Researchers are currently working on ways to improve diagnosis of Bartonella in these cases, but the nature of this stealth pathogen makes it difficult. When physicians are faced with patients suffering from endocarditis, risk factors for Bartonella species should be taken into account.
Risk Factors
The prevalence and incidence of Bartonella species can vary depending on geographic location. For example, you are more likely to contract B. quintana in South America, but B. henselae is commonly found in North America. Furthermore, certain regions of North America, such as the  southeastern United States, have more reported cases of Cat Scratch Disease than anywhere else in the United States. On top of this, seasonality and climate seem to affect the exposure rates of Bartonella species. Cases of bartonellosis have been reported around the world, however, so testing should be considered when the risk is high. There are common risk factors that contribute to contracting any type of bartonellosis:
southeastern United States, have more reported cases of Cat Scratch Disease than anywhere else in the United States. On top of this, seasonality and climate seem to affect the exposure rates of Bartonella species. Cases of bartonellosis have been reported around the world, however, so testing should be considered when the risk is high. There are common risk factors that contribute to contracting any type of bartonellosis:
- Outdoor or indoor exposure to fleas, biting flies, ticks, and other arthropods
- Working or living with pets or other animals
- People with naturally weaker immune systems (younger children, adolescents, aging adults)
- Cancer patients, people with immune disorders (lupus), or patients using immunosuppressants for therapeutic reasons (such as steroid therapy)
Diagnosis
Successful diagnosis of bartonellosis can be difficult due to the variety of atypical and non-specific symptoms that an infected person may have combined with the stealthy nature of the pathogens. Bartonella tends to be present at low levels and does not invoke a strong immune response. As Bartonella species cycle from blood to tissues, symptoms change along with the host’s immune response. Despite vast advances in diagnostic tools over the last century, conventional methods lack sensitivity. Multiple rounds of testing may be required before a positive result is achieved. Researchers are looking for new ways to detect Bartonella pathogens to save patients time, money, and frustration. Current methods include:
- Culturing methods are used to grow bacteria on a specifically formulated medium from samples such as blood and tissues from around the body. These methods have continued to evolve as knowledge of Bartonella species improves. Most recently, the use of liquid growth
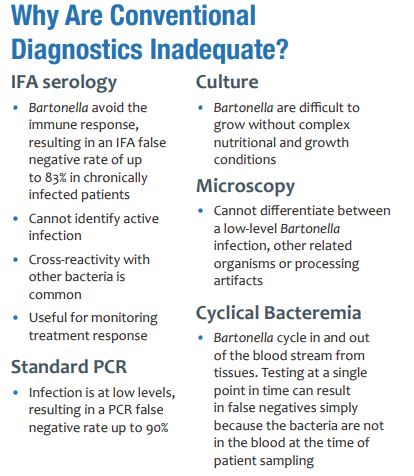 medium based on insect cell growth requirements has shown increased growth for multiple species. Successfully matching the strict growth requirements for Bartonella species makes it more likely to isolate and detect them. Patented BAPGM (Bartonella Alphaproteobacteria growth medium) is used in combination with ddPCR at Galaxy Diagnostics to maximize sensitivity.
medium based on insect cell growth requirements has shown increased growth for multiple species. Successfully matching the strict growth requirements for Bartonella species makes it more likely to isolate and detect them. Patented BAPGM (Bartonella Alphaproteobacteria growth medium) is used in combination with ddPCR at Galaxy Diagnostics to maximize sensitivity. - Serology refers to the use of the host immune response to detect the presence of Bartonella. When Bartonella is present, the immune system creates IgG and IgM antibodies to fight it. Methods such as immunofluorescence assays (IFA), enzyme immunoassays (EI), and enzyme-linked immunoassays (ELISA) use these antibodies to infer infection from Bartonella species. However, there are various limitations to serology. First, Bartonella species are able to evade the immune response because they are present in small amounts and engage in evasive strategies such as residing inside of red blood cells. Even if antibodies are present, it does not mean that Bartonella bacteria are actively present. There are also concerns with cross-reactivity between other bacteria that may lead to false positives. However, this method of detection is useful for monitoring treatment response because it is typically cheaper than other methods and provides a record of antibody amounts for a given period of time.
- PCR (polymerase chain reaction) is a method of detecting bacterial DNA in the blood and tissues of patients. By designing primers (small pieces of DNA) that match Bartonella DNA sequences at the genus or species level, it is possible to use an enzymatic reaction to amplify bacterial DNA that is present. This method confirms active infection, but this platform has difficulties as well. As mentioned earlier, Bartonella tend to infect at low levels. PCR has a chance of missing DNA if only a small amount is present. Furthermore, PCR can result in false positives if the wrong sequence of DNA is amplified. Positive results from PCR should always be verified by sequencing to ensure that it is Bartonella and not another bacteria. Galaxy Diagnostics’ Digital ePCR method combines a patented culture formulation with PCR and sequencing to maximize sensitivity.
Treatments
Bartonellosis is treated with antibiotic therapy, but the type(s) of antibiotics selected and the duration of treatment vary with the nature of the infection and must be evaluated by a physician on a case-by-case basis. Typically, acute infections can be treated with 10-21 days of antibiotic therapy. However, treatment failure can occur. Relapse of symptoms has been documented in numerous cases and chronic infections can take longer to treat.