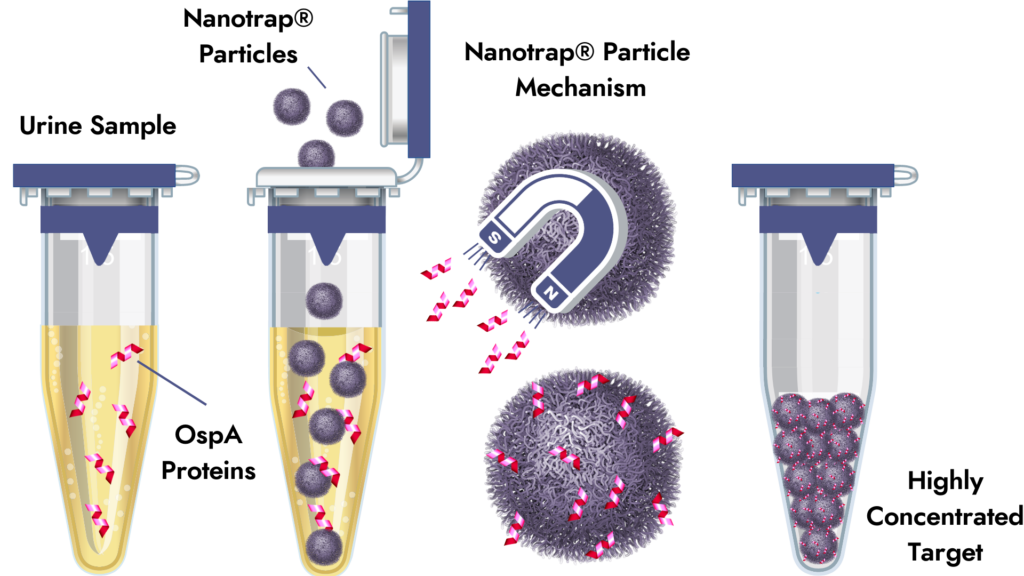
We recently announced the launch of the Lyme Borreliosis Nanotrap® Urine Test. In this post, Dr. Jennifer Miller discusses the Nanotrap® particle mechanism and the benefits of enhanced direct testing for Lyme Borrelia infections.
We were excited to talk about this new test with someone who personifies Galaxy’s “go beyond” principle daily. Dr. Jennifer Miller holds a doctorate in microbiology, immunology, and molecular genetics and has over 20 years of experience with vector-borne and zoonotic pathogens, including the Lyme disease spirochete Borrelia burgdorferi (aka Lyme Borrelia). As the vice president of research and development Galaxy Diagnostics, she leads clinical assay development and oversees our daily laboratory operations.
Galaxy Diagnostics scientists, including Dr. Miller, are dedicated to following the science to bring more sensitive and specific testing methodologies to market for hard-to-diagnose infections, like Lyme Borrelia. Working with our research partners, we are advancing diagnostics for flea and tick-borne diseases and driving research and discovery to support clinical utility and better patient care.
Check out our conversation with Dr. Miller and her perspective on the high potential value of Nanotrap® Urine Test for diagnosis of Lyme disease (aka Lyme borreliosis).
Q: The Nanotrap® Urine Test could be a game changer for clinical care of Lyme disease patients. Why is direct detection of Lyme Borrelia infections so important?
Early diagnosis and prompt treatment are critical. The best odds of success for eradicating infection occurs when diagnosis and treatment of Lyme disease occur in the early stage of infection (first 4-6 weeks). Unfortunately, current antibody tests, like standard two-tier testing (STTT) lack sensitivity during early-stage infection. ELISA and Western blot are tests, or assays, used to detect antibodies made by the immune system against Borrelia burgdorferi, the bacteria that causes Lyme disease. Early after Borrelia species infections, many patients have not yet mounted an antibody response. Direct detection of the bacteria would circumvent this technical problem, as an antibody response is not required for detection.
Antibody tests are difficult to interpret both early and late in infection because antibody responses only provide evidence of pathogen exposure but do not provide direct evidence of active infection. Historically, direct detection of Lyme Borrelia has been difficult because the bacteria are low-yield pathogens that do not replicate to high numbers anywhere within the body and may only be present at 1 organism per 10 ml of blood. This low abundance makes direct detection by PCR-based amplification challenging, as there is a lot more host or background DNA than Borrelia DNA. Direct detection is like trying to detect a needle in a haystack. Blood also contains inhibitory substances that can interfere with PCR-based amplifications. The ability of Nanotrap® particles to capture and concentrate small amounts of Borrelia antigen circumvents the limitations of both PCR and antibody testing.
Q: Our new test is called the Lyme Borreliosis Nanotrap® Urine Test. Why is urine a good sample to test for Lyme Borrelia infection?
Studies show that both mice and humans shed low numbers of Borrelia organisms and antigens into urine. Compared with blood draws, obtaining a urine sample is painless and non-invasive which can be a big advantage for children, the elderly, and those who dislike needles. Urine also has fewer inhibitory substances than blood that may interfere with detection of the target (protein or DNA).
Q: The target of the assay is the OspA antigen. What is OspA?
Outer Surface Protein A is a Lyme Borrelia-specific protein that is expressed on the outer surface of the bacterial membrane. OspA is a tick adhesin – it allows Borrelia to stick to the midgut of ticks so that the bacteria remain inside the tick during feeding. As for other bacterial virulence proteins, Lyme Borrelia differentially regulate OspA expression to help “sense” the environment around them–inside a tick, animal host, or in culture medium, blood, tissue, etc.
Q: What Borrelia species are detectable with this test?
Eurasian Lyme Borrelia species (senso lato) have different OspA serotypes from North American B. burgdorferi strains (senso stricto). The Nanotrap® particles bind to a narrow C-terminal OspA protein domain conserved across strains/species within both complexes of B. burgdorferi.
This finding was nicely demonstrated in a 2015 publication from researchers at George Mason University (Magni et al 2015, J. Transl Med). As this paper was published prior to the discovery of Borrelia mayonii, this species was not included in their assessment. More research is needed to address utility of Nanotrap® particle capture across newer species/strains.
Q: The Nanotrap® particles “capture and concentrate” OspA. What does this mean? Why is sample enrichment necessary?
Sample enrichment via antigen capture is necessary because B. burgdorferi organisms and their surface proteins are present at very low concentrations (i.e., 1 organism per 10 ml blood) in biological fluids, making direct detection a challenge (often called the “needle in a haystack conundrum”). Due to specialized chemistry, Nanotrap® particles have a specific affinity for OspA and will selectively bind to or “capture” it. High-speed centrifuges are then used to concentrate and recover OspA bound particles from large volumes of urine. Upon recovery, the resulting pellet is resuspended in a small volume of buffer for confirmation analyses.
Q: How does the Nanotrap® Urine Test methodology compare to antibody tests, like the CDC-recommended two-tiered testing?
The Nanotrap® Urine Test is a direct detection test, so it provides direct evidence of an active infection. The CDC standard two tier testing methodology and other antibody tests are indirect, as they measure antibody responses, which provide evidence of pathogen exposure but do not provide direct evidence of active infection.
There is no single “magic bullet” test that accurately diagnoses every instance, symptom or circumstance of a stealth, chronic low-yield infection, like Lyme Borreliosis. A battery of tests is often needed and should contain a combination of indirect and direct test methodologies to adequately cover an expected disease spectrum within a diverse population.
Q: It is recommended that the Nanotrap® Urine Test is ordered in conjunction with two-tiered testing. Why is this necessary?
Our internal validation data suggest that the Nanotrap® Urine Test detects Lyme Borrelia in patients missed by standard two-tier testing. Conversely, there were also a subset of samples reactive by two-tier testing, but not positive by the Nanotrap® Urine Test. Ongoing clinical utility studies will further delineate the specific disease stages and presentations best confirmed by the Nanotrap® Urine Test. As we have in the past, we will share these findings through peer-reviewed publication.
Q: What data are there to show that this test is effective for early Lyme disease? What about later stages and different disease presentations, like Lyme arthritis?
Magni and colleagues (2015, J. Transl. Med) were able to utilize the Nanotrap® Urine Test to accurately detect Lyme Borrelia in 24 of 24 early, acute Lyme disease samples. Accurate detection was also achievable across a swath of samples obtained from small numbers of patients exhibiting various later-stage disease presentations. Larger, more expansive clinical utility studies will further delineate all the disease stages and presentations detectable with the Nanotrap® Urine Test. We are working with academic research partners to conduct these studies now.
Q: What kind of research would you like to see that more precisely documents the clinical utility of the Nanotrap® Urine Test?
The most direct way to assess clinical utility is to test large numbers of defined, well-characterized samples belonging to each category (i.e., Lyme arthritis, Lyme carditis, neuroborreliosis) or population (i.e., acute, chronic, PTLDS) and compare the results obtained with the new test of interest (i.e., the Nanotrap® Urine Test) to those obtained with the standard-of-care reference, standard two-tier antibody testing.
Ideally, testing would utilize paired samples encompassing multiple matrices of interest (whole blood, serum, urine, and tissue) and would also involve comparison to widely utilized and well-characterized direct detection test modalities, such as qPCR. Obtaining the number and diversity of sample types needed for a robust clinical utility study remains challenging for many vector-borne diseases, including Lyme Borreliosis.
History: 10 Aug 2024 – updated Jen Miller title.
Interested in learning more about the Nanotrap® Urine Test? Please contact us by e-mailing contact@galaxydx.com or calling 919-313-9672.