Individuals exposed to fleas and ticks are at risk of polymicrobial infections, which can lead to complex multi-system disease. It is difficult and expensive to confirm which infections are present with currently available laboratory testing, so healthcare providers often must clinically diagnose patients based on symptoms and exposure risk. A new direct detection method, the multiplex ddPCR assay, will improve the efficiency and accuracy of direct testing for bartonellosis, babesiosis, and Lyme borreliosis, allowing healthcare providers and patients to be more confident in diagnoses.
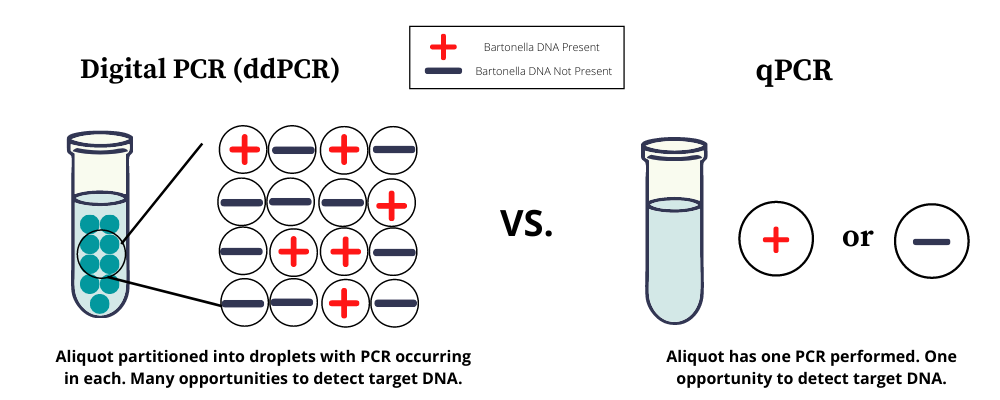
Galaxy Diagnostics launched the first and only commercially available droplet digital PCR (ddPCR) assay for Bartonella species infections earlier this year. Instead of performing a single reaction on a test sample, this powerful technology partitions the sample into approximately 20,000 droplets and analyzes each droplet for the presence of DNA. By reducing the potential number of PCR inhibitors in each reaction, the sensitivity of direct detection for low-abundance pathogens is greatly increased. As shown in published studies, ddPCR for Bartonella is up to 10x more sensitive than conventional PCR methods.
A recent publication in Pathogens co-authored by researchers from North Carolina State University and Galaxy Diagnostics demonstrates the potential for a multiplex ddPCR assay that can confirm Bartonella, Borrelia, and Babesia species infections simultaneously.
What is a multiplex test?
Multiplexing is utilized in diagnostic and research testing for many reasons. Rather than having to prepare multiple test samples for each target, the targets are simultaneously detected in a single reaction. This means less money and time, and a smaller sample is required to yield results.
Multiplex ddPCR is beneficial to the laboratory running the testing, but it also provides downstream benefits to the patient and healthcare provider. However, designing a reliable multiplex assay is more challenging than standard methods, especially for novel applications. Regulatory standards for clinical testing are also not well defined for innovative methods like those used at Galaxy Diagnostics, extending the timeline for optimization and validation of the assay.
Multiplexing the more sensitive direct detection test—ddPCR for flea- and tick-borne disease— would be significant though. Currently available methods, like standard PCR, have a high rate of false negatives for these elusive pathogens. Further, serology test that are considered the gold standard often yield results that are difficult to interpret in the context of chronic infection and possible coinfection. A more robust direct detection approach could better guide treatment and advance clinical research for the complex diseases associated with Bartonella, Borrelia, and/or Babesia infections.
The data from the publication in Pathogens showed that the multiplex ddPCR assay was able to detect 31 Bartonella species, 13 Borrelia species and 24 Babesia species including Theileria equi, T. cervi, and Cytauxzoon felis. Further, the test was able to detect two and three-pathogen coinfections.
Can I order the multiplex ddPCR test?
The multiplex ddPCR test is not yet available for clinical use. Bartonella ddPCR and Bartonella Digital ePCR™ are currently available through Galaxy Diagnostics though. The Bartonella Digital ePCR™ platform combines ddPCR with our proprietary sample enrichment medium, BAPGM™, to detect positives that may be otherwise missed due to low pathogen load in the sample.
We recommend following us here and on social media for the latest research and test offering updates. More information about flea- and tick-borne pathogens and our current test options can be found here.