A revolutionary test method to confirm the presence of Borrelia infection.
This urine-based test provides more sensitive direct detection for Borrelia burgdorferi infection at all stages of Lyme disease than standard methods.
- Identifies positive cases missed by conventional Two-Tiered Testing (TTT)
- Reduces concern for false positive results by direct detection of OspA antigen
- Confirms active infection with easy to collect urine samples
Some patients never develop Lyme disease antibodies
How it works
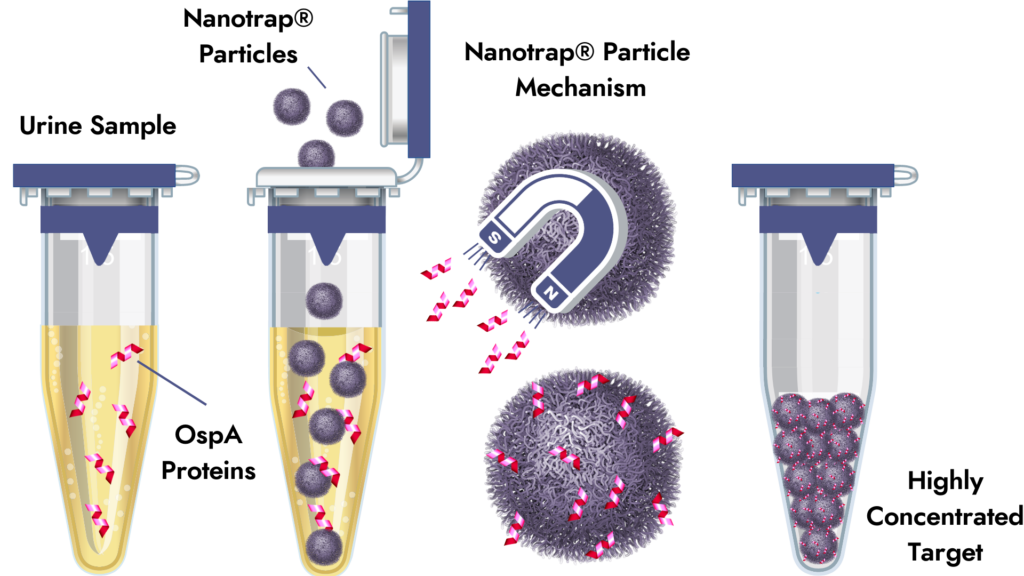
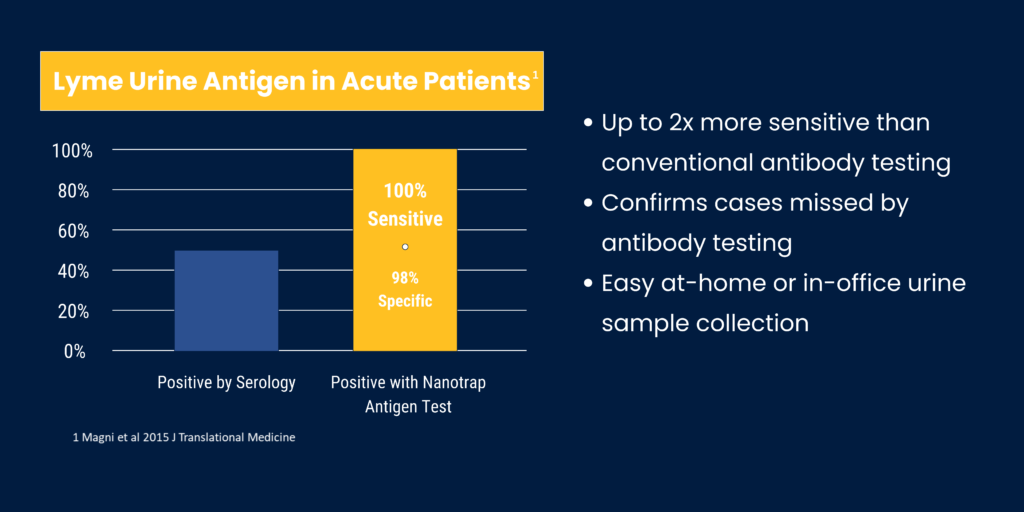
The Lyme Nanotrap® Urine Antigen Test provides more reliable confirmation of active Borrelia burgdorferi infection. Capture and concentration of Outer surface protein A in urine is confirmed by Western blot. Clinical utility data documented 100% sensitivity/98% specificity in 24/24 patients with EM rash and showed 41% positive rate in post-treatment patients monitored for persistent infection (Magni et al 2015 J Translational Medicine). Our proprietary reimbursement codes now reduce the out-of-pocket expense for patients.
Research
Prior research shows that a small amount of Outer surface protein A (OspA) antigen is detectable in the urine of Lyme disease patients. Nanotrap® particle sample enrichment greatly increases the likelihood of direct detection of these low abundance proteins. Based on preliminary studies, Nanotrap® sample enrichment can increase the concentration of low-abundance targets by 10,000-fold.
Data
Published data shows the Nanotrap® Antigen Test is very effective for confirmation of early stage Lyme borreliosis in patients with EM rashes (24/24). Galaxy validation data (unpublished) shows it is able to detect pathogen presence in patients with negative TTT results. Further research is needed to confirm clinical utility for other presentations of Lyme borreliosis, including Lyme arthritis, Lyme carditis, and neuroborreliosis.
Experts recommend combining direct detection test methods with indirect antibody testing to maximize the diagnostic data for low abundance infections, like Lyme borreliosis.
➜ Order BBB Direct Detect + Lyme Borrelia Direct Detect
At Galaxy, we recommend the Lyme Borrelia (Nanotrap) Direct Detect assessing the current presence of a Borrelia infection. Concentrations of Lyme Borrelia are so low in the blood that a blood draw is unlikely to capture the pathogen in the test tube.
Why include Borrelia in the BBB assay? While the species associated with Lyme Borrelia often hide in tissues and don’t free-circulate in high copy numbers in blood, the species associated with Relapsing Fever Borrelia do replicate to high numbers in the blood.
As a result, combining the BBB assay with the Nanotrap® urine antigen test for Lyme provides optimal coverage for the top flea and tick-borne infections at the genus level.
FAQS
What Borrelia species can be detected?
The Nanotrap® test is targeting the OspA protein, which can be found on the surface of Borrelia species belonging to both major groups (sensu stricto and sensu lato). The test will only determine if OspA is present, not which Borrelia species was detected.
How do I order the Nanotrap®test?
A licensed healthcare provider needs to request an account before ordering a urine kit through our website. The kit can be shipped to the provider’s office, or directly to the patient. It will include a urine cup, the necessary paperwork, specimen collection instructions, and packing/shipping materials.
How much urine is needed?
The Nanotrap® test requires at least 40 mL of urine. The cup provided in Galaxy’s specimen collection kit has a sticker marking the required amount. We recommend collecting as much as possible in case some sample is spilled during transit.
How should the urine be shipped?
We recommend shipping the urine the same day it is collected. It should be shipped overnight with a completely frozen ice pack. Please do not ship on Fridays.
See our Specimen Collection Instructions for details.