The classic biological definition of parasitism is “a symbiotic relationship where one member, the parasite, gains benefits that come at the expense of the host”. This means only the unwelcome guest gains nutritional and/or protective benefits while the host’s health suffers. However, emerging research indicates low-level infections could be beneficial because of the complex relationship between parasites and the mammalian immune system.
The term “parasites” encompasses a broad range of macroscopic and microscopic species that acquire nutrients from blood or the tissues (liver, skin, intestines, etc.) located throughout the human and/or animal body. Despite their dependence on a host, many parasites can live in the environment or within a vector (such as a mosquito, tick, flea, etc.) long-term at certain life stages. However, they cannot successfully grow and reproduce without encountering an appropriate host at some point in their lifecycle.
Because of these intricate lifecycles that have developed, many evolutionary biologists believe that humans and their parasitic counterparts co-evolved over many years. Cheaper, faster genome sequencing that has reached the market in recent years has allowed researchers to test this hypothesis by diving deeper into the genetic makeup of parasites.
Based on these studies, researchers estimate that genes responsible for surface proteins (proteins that are exposed to the immune system) evolved earlier than others in the genome and often vary greatly between closely related species. The ability to avoid or modify the host immune response provides a clear advantage for parasites, and varied effects on the host.
The Center for Disease Control (CDC) suggests that millions of people around the world are burdened by the effects of parasites. According to the CDC, there are three classes of parasites that cause most human health problems:
Ectoparasites
Common examples of ectoparasites include lice, ticks, fleas, and mites. These multicellular organisms can attach and/or burrow into the skin of a host for an extended time leading to irritation and occasionally a secondary bacterial or fungal infection. In immunocompromised hosts, the symptoms may be more severe.
Sarcoptes scabiei, the mite responsible for scabies in humans, causes itchiness and irritation when females burrow into the skin and lay eggs. In dogs and cats, flea bites can cause allergic dermatitis which often results in hair loss as well as a secondary skin infection sometimes.
Despite causing damage to the host’s skin, the most impactful consequences of ectoparasite infections come from the microscopic bacteria and parasites that they can carry and transmit. These pathogens are hard to detect through standard testing methods and often cause non-specific symptoms that make them hard to diagnose. Bartonella, Borrelia, and Babesia species are tick-borne pathogens that cause a wide range of acute and chronic symptoms in humans and animals.
Please view our educational webpages, From Cat Scratch Disease to Bartonellosis and From Lyme disease to Borreliosis, to learn more about transmission, symptoms, and testing for Bartonella and Borrelia species.
Helminths
The World Health Organization estimates that 880 million children around the world require treatment for soil-transmitted helminths. Helminths are multicellular, parasitic worms that are visible to the naked eye in their adult stage. Their eggs and larvae are transmitted to humans when contaminated soil, water, or infected food is ingested. Most cases are reported in areas of poverty and/or poor sanitation.
Intestinal worms, like roundworms (Ascaris lumbricoides) and hookworms (Ancylostoma duodenale, Necator americanicus) develop into adults by stealing nutrients from the host’s intestinal tract. Some parasitic worms, like liver flukes or Toxocara species, prefer to travel to other organs such as the liver, eyes, or central nervous system where they can access a rich blood supply. Once adulthood is reached, they can shed thousands of eggs daily.
Helminth infections (helminthiases) can cause diarrhea, abdominal pain, and loss of appetite, but are typically not deadly. The severity of symptoms is typically related to the burden of parasites that an individual is carrying. The most detrimental consequences of these infections seem to be the declines in cognitive abilities as a result of malnourishment. In a 2018 study of children in Ethiopia, researchers found that students with intellectual disabilities were more likely to be infected by parasites than students without such disabilities.
Protozoans
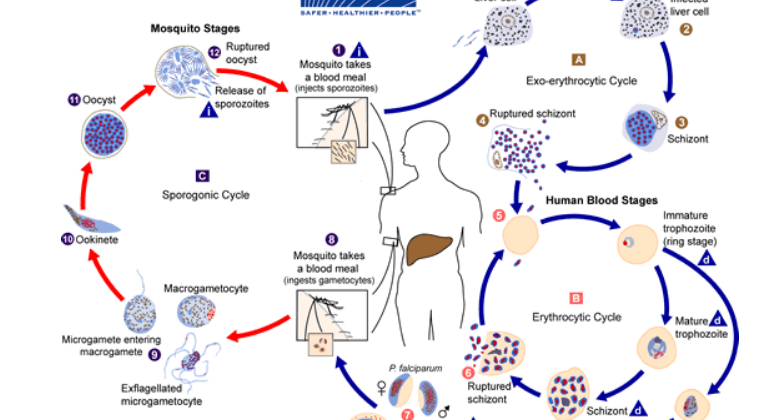
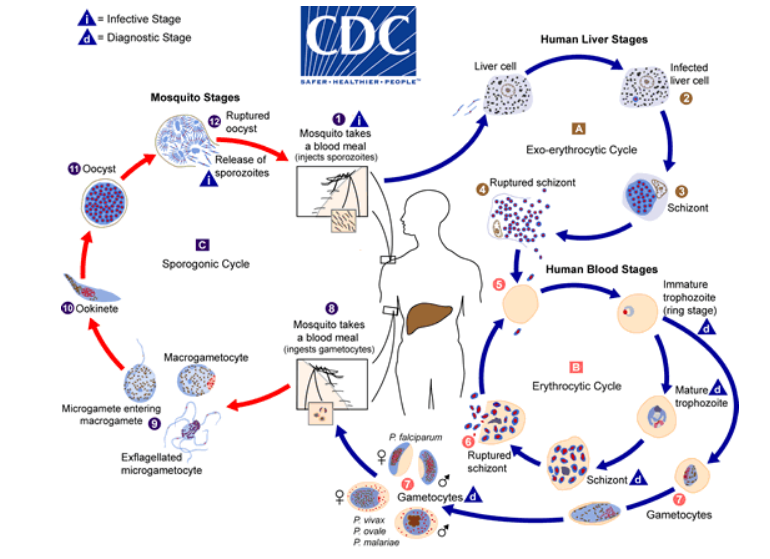
Protozoans are unicellular organisms that are spread to humans via infected soil, water, and food. They can also be transmitted via vectors such as ticks, mosquitoes, and triatomines (kissing bugs). In this post, we will focus on Babesia and Plasmodium species.
Babesia species are tick-borne parasites. Unlike many other familiar tick-borne pathogens, they are not bacteria. Babesia species, like Babesia microti and Babesia duncani, invade red blood cells where they acquire nutrients required for maturation and cell division.
Plasmodium includes many species, but only five of the species cause the disease malaria in humans. The parasites are transmitted by mosquitoes and have a complex lifecycle in the mosquito and the human host.
How could parasites be beneficial if they are responsible for so many health problems?
Despite all of these terrible diseases, some people with autoimmune conditions from developed nations infect themselves with parasites on purpose. Why? As part of the hygiene hypothesis, it may be that these pathogens have some beneficial effects on the immune system that has been lost in modern living.
One theory is that as these worms co-evolved with humans they developed a niche role in training the human immune system. Without their modulating influence the immune system can develop in unhelpful ways, generating autoimmune diseases or being unable to respond to pathogens effectively.
The effects on the immune system are complex. In many cases, the effects on the human body involve multiple pathogens. Humans and primates with certain parasitic worms are less likely to become infected with malaria and other pathogens. Exactly how the immune system is modulated is unknown, but measurable changes are seen in T-cells and cytokines.
Yet when one primate or person is infected with multiple species of worms these benefits are reduced. When people are infected with high numbers of worms, the benefits are also reduced. It may be that these large infections cancel out the immune modulating effects and lead to the kind of inflammation that is more typical in any other pathogenic infection and also more typical in autoimmune diseases.
Conclusion
Microbes that are today seen as dirty or even experienced as pathogenic (disease causing) may not have caused disease in their historic niche, in fact, they may have even been health promoting. However, the solution is not as simple as just infecting people living modern lifestyles with these microbes.
While a microbe may be health-promoting in people living traditional lifestyles, that may be because of its balance with an overall microbiome that differs from people living modern lifestyles. Discovering how to use microbes, including parasites, therapeutically in the radically different microbiome of modern life is a complex problem.
Without the traditional microbiome to keep their population at a suitable level, they may drop under or surge over the number of individuals needed for optimal health. It may be that identifying proteins they produce and turning those into medications may be more successful than infection with live parasites. Continued research on these parasites and the individuals exposed to them is needed.
References
Scutti, S. (2019, March 29). Teen dies of tapeworm larvae infestation in the brain. CNN.com. Retrieved from: https://www.cnn.com/2019/03/28/health/brain-parasites-case-study/
Mabbott, N. A. (2018). The influence of parasite infections on host immunity to co-infection with other pathogens. Frontiers in Immunology, 9, 2579. doi:10.3389/fimmu.2018.02579 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6237250/
Patient Centered Care Advocacy Group. (2016). Lyme bacteria hides inside parasitic worms, causing chronic brain diseases. PRNewsWire.com. Retrieved from: https://www.prnewswire.com/news-releases/lyme-bacteria-hides-inside-parasitic-worms-causing-chronic-brain-diseases-300270742.html
Haque, R. (2007). Human intestinal parasites. Journal of Health, Population and Nutrition, 25(4), 387-391. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2754014/
Fentahun, A. A. et al. (2019). Intestinal parasitic infections and associated factors among mentally disabled and non-disabled primary school students, Bahir Dar, Amhara regional state, Ethiopia, 2018: A comparative cross-sectional study. BMC Infectious Diseases, 19, 549. doi:10.1186/s12879-019-4165-2 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6588938/
Centers for Disease Control and Prevention. (2016). About parasites. Retrieved from: https://www.cdc.gov/parasites/about.html
Jackson, A. P. (2015). The evolution of parasite genomes and the origins of parasitism. Parasitology, 142(Suppl 1), S1-S5. doi:10.1017/S0031182014001516https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4413782/
Baggaley, K. (2017). Scientists are trying to treat autoimmune diseases with intestinal worms. Popular Science. July 28, 2017. https://www.popsci.com/can-intestinal-worms-treat-autoimmune-disease/