Harnessing the Power of Sample Enrichment
Slow-growing, immune-evasive pathogens present a particular challenge for scientists developing diagnostics for confirmation of infection. These elusive microbes often fly under the radar, evading direct and indirect detection using standard testing methods. From a clinical perspective, documentation of low abundance infection presents a significant false negative problem and may result in misdiagnosis of disease.
At Galaxy Diagnostics, we specialize in the development of diagnostic methods that harness the power of sample enrichment technologies and increase the sensitivity of direct detection. By enriching or partitioning the sample, we have developed direct testing methods that go beyond the limits of detection.
Our scientists have a deep understanding of flea and tick-borne diseases, allowing us to develop and optimize assays that begin with the pathobiology of the target microbe in mind. Advanced sensitivity and high specificity for low abundance pathogens is necessary to ensure early diagnosis of infections that too often go under-diagnosed and risk later presentation as more severe disease.
We use three advanced technologies to enhance detection of low abundance infections.
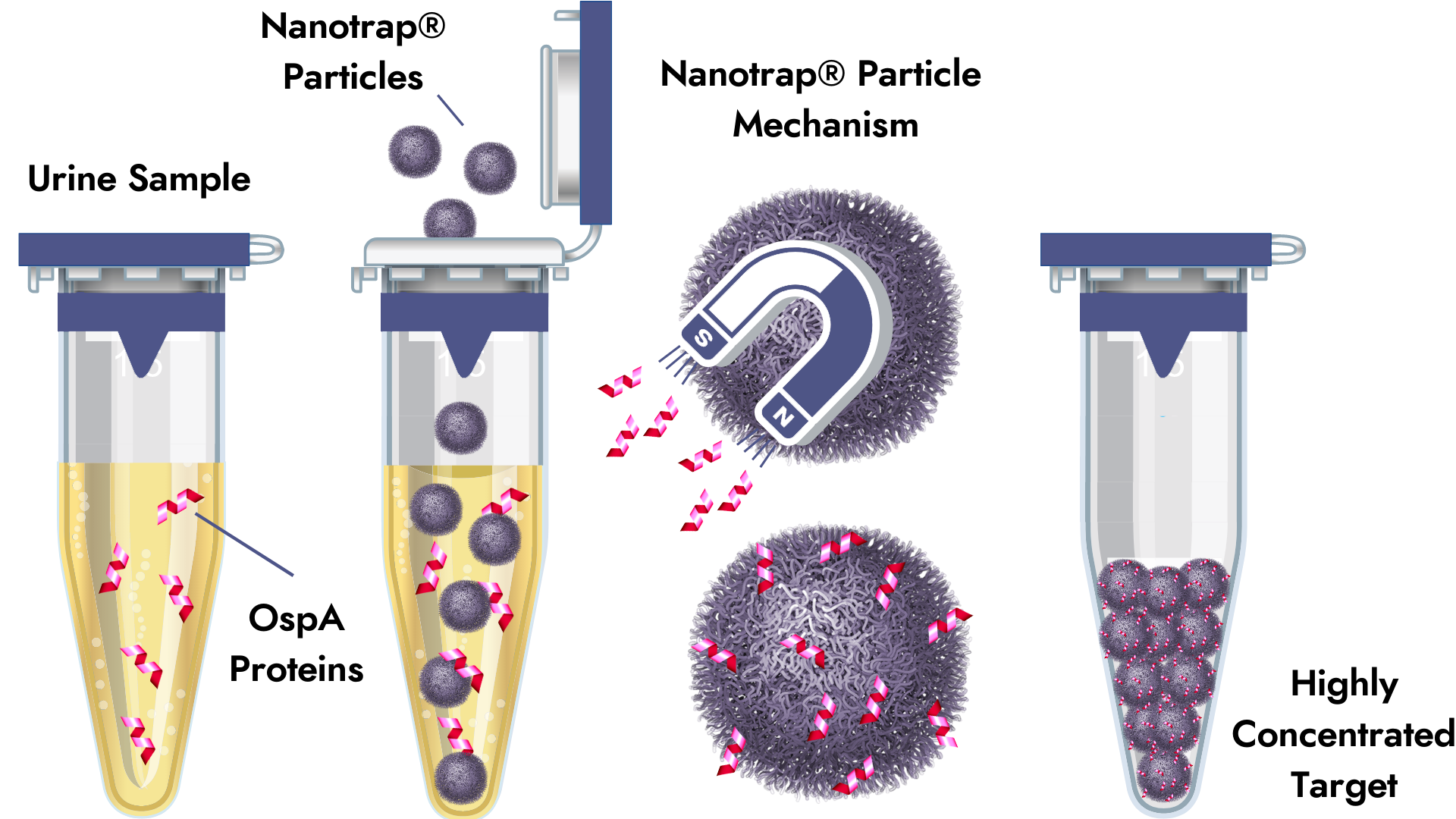
Nanotrap® Antigen Capture
Nanotrap® particles selectively capture and concentrate target proteins, increasing the sensitivity of the diagnostic test method. The inside of the nanoparticle binds a specific segment of a target protein, like a magnet, while excluding inhibitory compounds. After capture, the target proteins are concentrated and then confirmed by Western blot or another highly specific confirmation method. Direct detection of protein biomarkers in urine provides a reliable means of confirming infections that cannot be detected in blood.
Our Lyme Borreliosis Nanotrap® Urine Test uses particles that target OspA in urine. Published data shows that the Nanotrap® Urine Test is very effective for confirmation of early-stage Lyme borreliosis in patients with EM rashes (24/24).
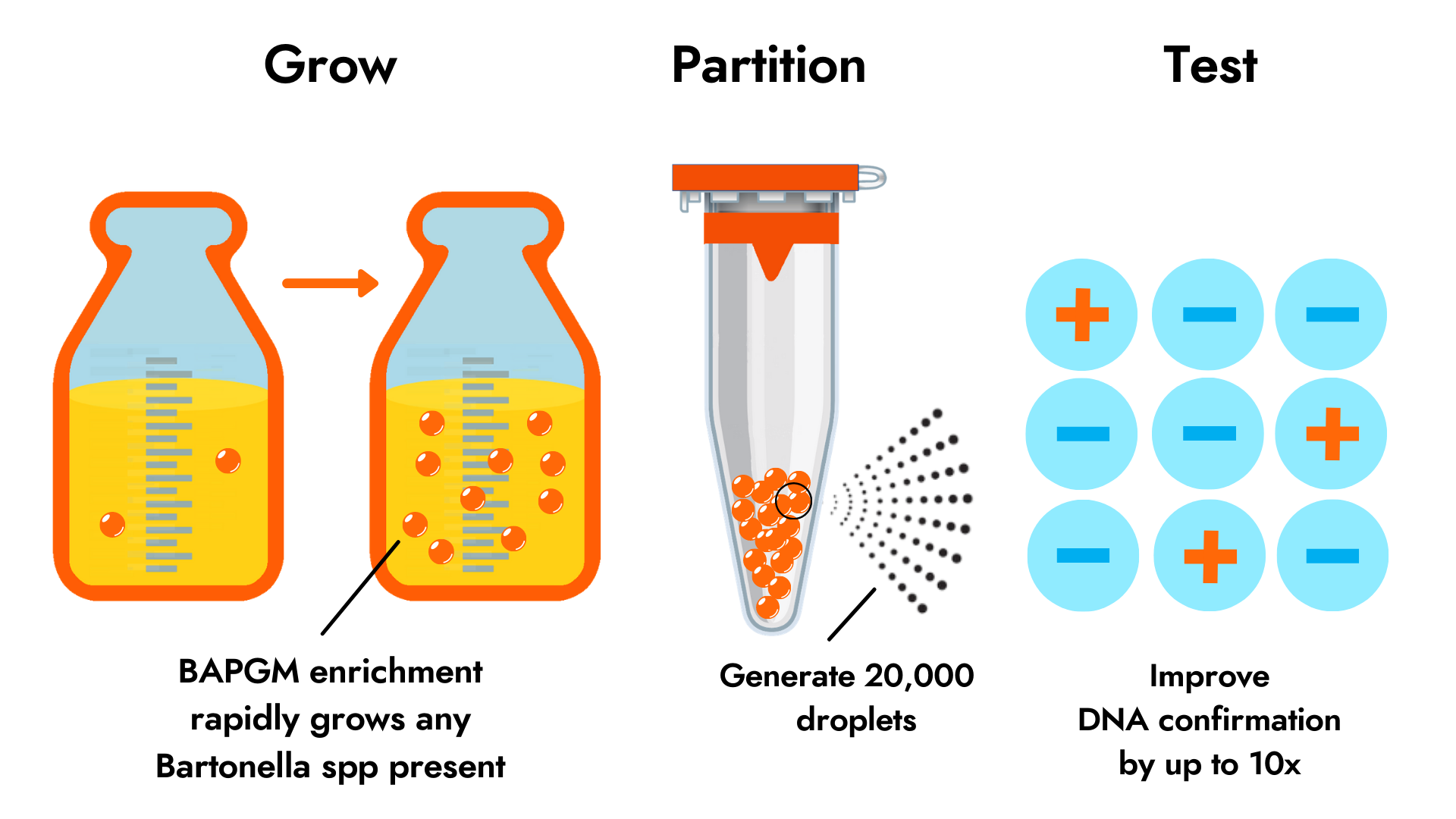
Sample Partitioning using ddPCR
Droplet digital PCR (ddPCR) overcomes the challenge of detecting low abundance pathogens in patient samples by partitioning each test sample into 20,000 droplets and performing PCR on each droplet, producing a positive signal each time target DNA is detected. This process reduces the background noise from host DNA, significantly increasing the chances of detecting small amounts of target DNA. We offer ddPCR testing for more sensitive detection of Bartonella species infection in a range of sample types. Published research suggests that Bartonella species ddPCR is up to 10 times more sensitive than qPCR.
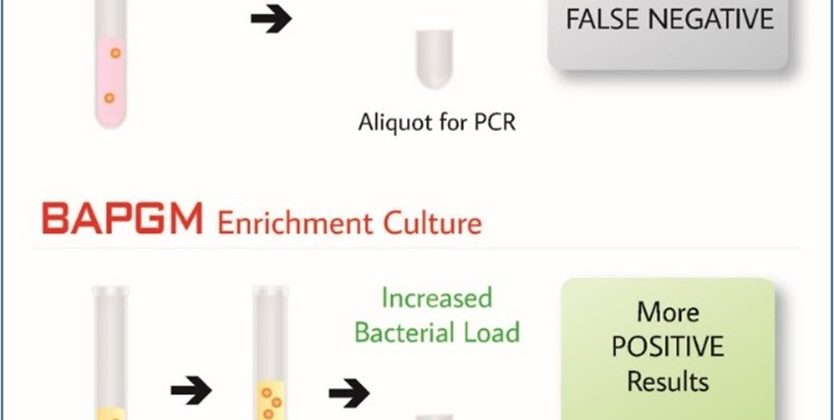
BAPGM™ (Bartonella Alpha-Proteobacteria Growth Medium) Enrichment
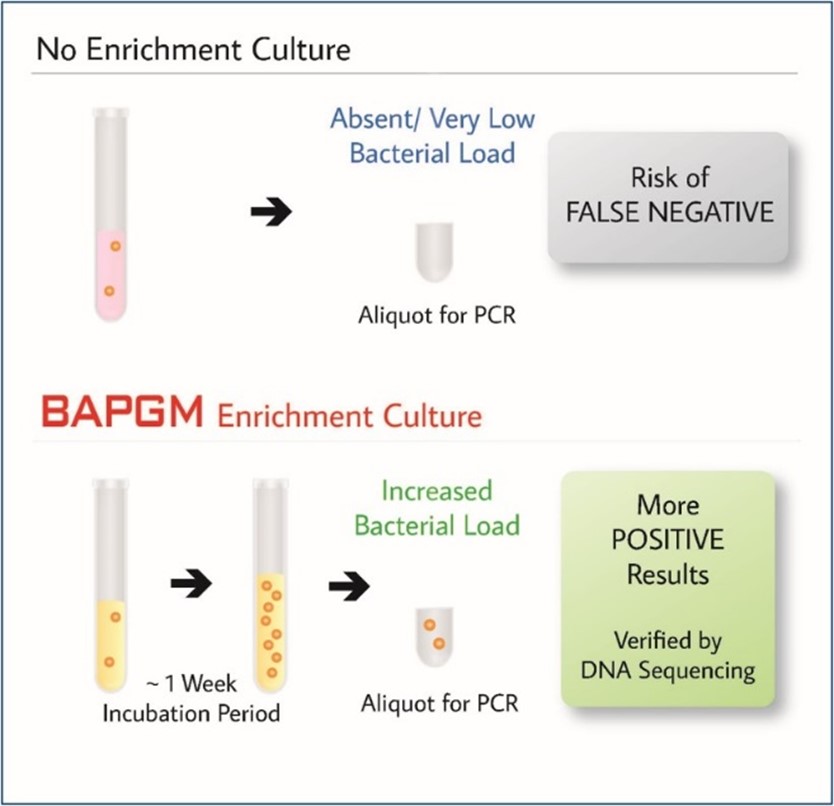
BAPGM™ is a patented growth medium developed at North Carolina State University by Dr. Breitschwerdt (Galaxy cofounder and chief scientific officer) and Dr. Ricardo Maggi (cofounder and chief technical officer). BAPGM™ enrichment is the first technology that we commercialized at Galaxy Diagnostics. This proprietary growth medium is specifically formulated to meet the growth requirements of Bartonella species, but has also been demonstrated to effectively grow a range of fastidious microbes.
Our Bartonella Digital ePCR™ test panel combines BAPGM™ enrichment with ddPCR to enhance direct detection of Bartonella species infections. One-week incubation in BAPGM™ increases the bacterial load of live organisms present in the sample up to detectable levels, increasing sensitivity of confirmation ddPCR by almost 30%.